Immune aging impairs muscle regeneration via macrophage-derived anti-oxidant selenoprotein P
- PMID: 40681871
- PMCID: PMC12373998
- DOI: 10.1038/s44319-025-00516-3
Immune aging impairs muscle regeneration via macrophage-derived anti-oxidant selenoprotein P
Abstract
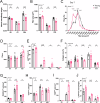
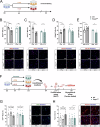
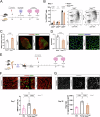
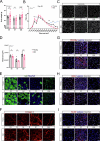
Muscle regeneration is impaired with aging, due to both intrinsic defects of muscle stem cells (MuSCs) and alterations of their niche. Here, we monitor the cells constituting the MuSC niche over time in young and old regenerating mouse muscle. Aging alters the expansion of all niche cells, with prominent phenotypes in macrophages that show impaired resolution of inflammation. RNA sequencing of FACS-isolated mononucleated cells uncovers specific profiles and kinetics of genes and molecular pathways in old versus young muscle cells, indicating that each cell type responds to aging in a specific manner. Moreover, we show that macrophages have an altered expression of Selenoprotein P (Sepp1). Macrophage-specific deletion of Sepp1 is sufficient to impair the acquisition of their restorative profile and causes inefficient skeletal muscle regeneration. When transplanted in aged mice, bone marrow from young WT mice, but not Sepp1-KOs, restores muscle regeneration. This work provides a unique resource to study MuSC niche aging, reveals that niche cell aging is asynchronous and establishes the antioxidant Selenoprotein P as a driver of age-related decline of muscle regeneration.
Keywords: Aging; Macrophages; Selenoprotein P; Skeletal Muscle Regeneration.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. DHH, JB, JG, PM, AG, OM, GJ, SL, RM, FLG, and BC declare no competing interests. EM, PS, and JNF are employees of Société des Produits Nestlé SA.
Figures



References
-
- Burk RF, Hill KE, Motley AK (2003) Selenoprotein metabolism and function: evidence for more than one function for selenoprotein P. J Nutr 133:1517s–1520s - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

