Informed consent in genetic and genomic studies in Sub-Saharan Africa: a systematic review of bioethical issues
- PMID: 40682008
- PMCID: PMC12275316
- DOI: 10.1186/s12910-025-01170-z
Informed consent in genetic and genomic studies in Sub-Saharan Africa: a systematic review of bioethical issues
Abstract
Background: International collaboration on genetic/genomic studies has expanded dramatically. Informed consent remains a cornerstone for protecting participant autonomy. Over the past few decades, guidelines, recommendations, and theoretical discussions have been published on the informed consent process in cross-cultural contexts, particularly in Africa. These resources suggest best practices-ranging from assessing participant comprehension to engaging local communities. However, it remains unclear how well the current practices address the full range of informed consent challenges encountered by the stakeholders.
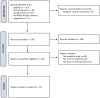
Methods: We performed a systematic literature review of empirical studies that explored stakeholders' perspectives, primarily research participants and researchers, on informed consent in the context of international genetic/genomic research in countries of Africa. We followed the PRISMA guideline and used thematic analysis for data synthesis and analysis.
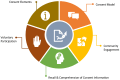
Results: Twenty-four relevant articles were identified and reviewed for five bioethical issues: (1) comprehension of consent information, (2) voluntary participation, (3) consent elements, (4) consent model, and (5) community engagement. Different levels of understanding and recall over different consent elements, and multiple factors associated with comprehension were observed. Voluntary participation could be influenced by misconception, monetary and healthcare compensation, and previously established trust with the research team. Many participants show less interest in certain consent elements, such as biobanking, data sharing, and future use, but more in the section on benefits. Returning results had potential advantages in building trust between participants and the research team; however, the most appropriate way to return results is under discussion. There was also conversation over consent models (broad, tired, and dynamic) on their balance between autonomy and practicality. Finally, many articles ascertained the value of community engagement and encouraged researchers to consider local cultural beliefs, social stigma, and decision-making habits when designing the consent process.
Conclusion: We observed an increased interest in the ethical conduct of the informed consent process under international genetics/genomics studies in recent decades. Awareness and attention to these issues are needed to develop more appropriate consent strategies that could further safeguard the rights and welfare of local participants and support the smooth conduct of research.
Keywords: Ethics; Genetics and genomics research; Informed consent; Sub Saharan-Africa.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous