Brain functional connectivity correlates of autism diagnosis and familial liability in 24-month-olds
- PMID: 40682020
- PMCID: PMC12275292
- DOI: 10.1186/s11689-025-09621-9
Brain functional connectivity correlates of autism diagnosis and familial liability in 24-month-olds
Abstract
Background: fcMRI correlates of autism spectrum disorder (ASD) diagnosis and familial liability were studied in 24-month-olds at high (older affected sibling) and low familial likelihood for ASD.
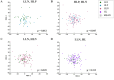
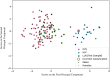
Methods: fcMRI comparisons of high-familial-likelihood (HL) ASD-positive (HLP, N = 23) and ASD-negative (HLN, N = 91), and low-likelihood ASD-negative (LLN, N = 27) 24-month-olds from the Infant Brain Imaging Study (IBIS) Network were conducted, employing object oriented data analysis (OODA), support vector machine (SVM) classification, and network-level fcMRI enrichment analyses.
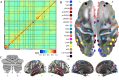
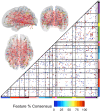
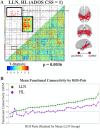
Results: OODA (alpha = 0.0167, 3 comparisons) revealed differences in HLP and LLN fcMRI matrices (p = 0.012), but none for HLP versus HLN (p = 0.047) nor HLN versus LLN (p = 0.225). SVM distinguished HLP from HLN (accuracy = 99%, PPV = 96%, NPV = 100%), based on connectivity involving many networks. SVM accurately classified (non-training) LLN subjects with 100% accuracy. Enrichment analyses identified a cross-group fcMRI difference in the posterior cingulate default mode network 1 (pcDMN1)- temporal default mode network (tDMN) pair (p = 0.0070). Functional connectivity for implicated connections in these networks was consistently lower in HLP and HLN than in LLN (p = 0.0461 and 0.0004). HLP did not differ from HLN (p = 0.2254). Secondary testing showed HL children with low ASD behaviors still differed from LLN (p = 0.0036).
Conclusions: 24-month-old high-familial-likelihood infants show reduced intra-DMN connectivity, a potential neural finding related to familial liability, while widely distributed functional connections correlate with ASD diagnosis.
Keywords: Default mode network; Familial; Functional connectivity; Infant; MRI.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study protocol was reviewed and approved by the internal review boards of Washington University School of Medicine, IRB IDs 201103140 and 201301110, the University of Washington, IRB IDs 12317 and STUDY00012991, The Children’s Hospital of Philadelphia, IRB ID 07-005689, and the University of North Carolina at Chapel Hill, IRB ID 05-2293. Informed consent was signed by all study participants. Competing interests: Dr. Robert McKinstry serves on the advisory board of Nous Imaging, Inc. and receives funding for meals and travel from Siemens Healthineers and Philips Healthcare. Abraham Z. Snyder is a consultant for Sora Neuroscience, LLC. All other authors report no financial relationships with commercial interests.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

