Rapid diagnostic imaging and targeted immunotoxin delivery in aggressive prostate cancer using CEACAM5-specific nanobodies
- PMID: 40682047
- PMCID: PMC12275256
- DOI: 10.1186/s12951-025-03600-x
Rapid diagnostic imaging and targeted immunotoxin delivery in aggressive prostate cancer using CEACAM5-specific nanobodies
Abstract
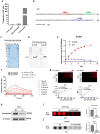
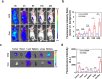
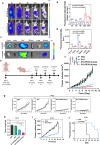
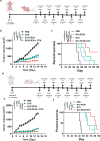
Aggressive variant prostate cancer (AVPC) originates from metastatic prostate cancer (mPCa) following androgen receptor-targeted therapies, leading to diverse pathological subtypes, notably castration-resistant prostate cancer (CRPC). Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5), is consistently expressed across AVPC phenotypes, including neuroendocrine prostate carcinoma (NEPC) and double-negative prostate carcinoma (DNPC), which are significant subtypes of CRPC, making it a promising therapeutic target. In this study, A high-affinity nanobody, B12, specific to CEACAM5, was discovered through phage library screening. B12 exhibited robust binding capabilities, enhanced tumor accumulation, and effective tissue penetration, facilitating rapid in vivo imaging of AVPC. The conjugation of B12 with PE38 to create the immunotoxin B12-PE38 showed significant anti-tumor activity in AVPC xenograft models, including one that mimics bone metastasis. When B12-PE38 was combined with docetaxel, it elicited enhanced tumor inhibitory effects, effectively inhibiting tumor progression. This study underscores CEACAM5 as a target for precise imaging and targeted therapy in AVPC, introducing novel diagnostic and therapeutic strategies for a disease that currently faces a dearth of effective treatment options due to the scarcity of well-defined targets.
Keywords: Aggressive variant prostate cancer; CEACAM5; Immunotoxin; Nanobodies; Phenotype transformation; Rapid imaging.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The research was approved by the Ethics Committee of the Fifth Affiliated Hospital of Southern Medical University (2024MNWK-K-001), the Shenzhen People’s hospital (AUP-230224-LZJ-543-01) and Nanchang University (BR/AF/SG-04/1.0-202112). Consent for publication: All subjects have written informed consent. Competing interests: The authors declare no competing interests.
Figures



References
-
- Paolieri F, et al. Front-line therapeutic strategy in metastatic hormone sensitive prostate cancer: an updated therapeutic algorithm. Clin Genitourin Cancer. 2024;22(4):102096. - PubMed
-
- Belderbos BPS, et al. Novel treatment options in the management of metastatic castration-naïve prostate cancer; which treatment modality to choose? Ann Oncol. 2019;30(10):1591–600. - PubMed
-
- Davis ID, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–31. - PubMed
-
- Wang Y, et al. Mechanisms of enzalutamide resistance in castration-resistant prostate cancer and therapeutic strategies to overcome it. Br J Pharmacol. 2021;178(2):239–61. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

