Prospective evaluation of surgical treatment of liver metastasizing pancreatic cancer - ScanPan study protocol
- PMID: 40682051
- PMCID: PMC12275338
- DOI: 10.1186/s12893-025-02983-w
Prospective evaluation of surgical treatment of liver metastasizing pancreatic cancer - ScanPan study protocol
Abstract
Introduction: Patients with pancreatic ductal adenocarcinoma (PDAC) have a dismal prognosis. The majority of patients are diagnosed at an advanced stage, and for these patients, the only possible treatment is palliative chemotherapy. There are increasing data from retrospective studies indicating that a subgroup of patients with liver-limited metastases may benefit from surgical treatment of liver metastases. However, there is a need for prospective trials.
Objective: The aim of this study is to prospectively investigate the safety and feasibility of surgically treating patients who are resectable, including those with borderline venous resectable, histopathologically confirmed PDAC, and histopathologically or radiologically confirmed liver metastases.
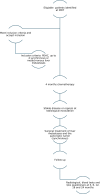
Methods: Five Swedish and one Finnish hepatopancreaticobiliary (HPB) centre will participate. Eligible patients will be identified at regional multidisciplinary conferences (MDTs). Before inclusion, they will undergo computed tomography (CT), magnetic resonance imaging (MRI, ) and (positron emission tomography computed tomography)PET-CT to rule out extrahepatic metastases. To be included, patients will have to have four or fewer liver metastases, which must be no larger than 5 cm for patients planning for resection and no larger than 2 cm for patients planning for ablation. The metastases may be either synchronous or metachronous. Patients will undergo four months of chemotherapy before surgical treatment (either resection or ablation), and postoperatively, they will undergo two months of chemotherapy. For those with synchronous metastases, resection of the pancreatic tumour will be performed. Follow-up will be performed over two years postoperatively with regular CT scans and assessments of quality of life.
Conclusions: In conclusion, this trial will provide increased knowledge concerning whether surgical treatment of liver metastases from pancreatic cancer can result in improved survival.
Clinical trial number: Clinical.
Trials: gov (NCT05271110), registered February 26th 2022.
Keywords: Ablation; Liver metastases; Pancreatic cancer; Resection.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Latenstein AEJ, et al. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer. 2020;125:83–93. - PubMed
-
- Hackert T, et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 2017;43(2):358–63. - PubMed
-
- Schwarz C, et al. Metachronous hepatic resection for liver only pancreatic metastases. Surg Oncol. 2020;35:169–73. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical