Early prediction of gestational diabetes mellitus based on systematically selected multi-panel biomarkers and clinical accessibility-a longitudinal study of a multi-racial pregnant cohort
- PMID: 40682053
- PMCID: PMC12275388
- DOI: 10.1186/s12916-025-04258-w
Early prediction of gestational diabetes mellitus based on systematically selected multi-panel biomarkers and clinical accessibility-a longitudinal study of a multi-racial pregnant cohort
Abstract
Background: Early identification of high-risk women is critical for preventing gestational diabetes mellitus (GDM). We aimed to improve early prediction of GDM using multiple panels of cardiometabolic biomarkers assessed in early and mid-pregnancy, considering clinical accessibility.
Methods: In a US study of 2802 pregnant individuals, we assessed 91 cardiometabolic biomarkers at 10-14 (random blood) and 15-26 (fasting) gestational weeks (GW) in 107 GDM cases and 214 controls. Candidate biomarkers were categorized by clinical accessibility from high to low: group I (clinically accessible tests like HbA1c, lipids), group II (clinically accessible biomarkers upon request like insulin-like growth factor (IGF) axis markers, adipokines), and group III (specialty lab-required targeted metabolomics: amino acids (AAs) and phospholipid fatty acids (FAs)). At each visit, we constructed a full model incorporating all candidate biomarkers and conventional predictors. We built alternative models utilizing different groups of biomarkers considering clinical accessibility. Variable selection was performed to retain variables with a p value < 0.10 for a parsimonious model. Model performance was evaluated by area under receiver operating characteristics curve (AUC), proportion of cases followed (PCF, %) and proportion needed to follow (PNF, %), and decision curve analysis.
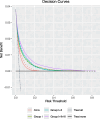
Results: A full model comprising conventional predictors, clinical and non-clinical cardiometabolic biomarkers, and metabolomic markers achieved the highest discriminative accuracy (AUC: 0.842 at 10-14 GW, 0.829 at 15-26 GW). The addition of novel biomarkers increased PCF and decreased PNF, suggesting increased clinical utility. For example, at 10-14 GW, 69.5% of GDM cases are expected to be detected from women whose risk is above the 80% percentile estimated by the full model vs. 49.1% by the conventional model. Additionally, 46.1% of women identified as being at the highest risk by the full model are expected to account for 90.0% of GDM cases vs. 71.1% by the conventional model. Decision curve analysis showed that models incorporating novel biomarkers performed better than the conventional model including glucose, and the full model at 10-14 GW had the highest net benefit, overall.
Conclusions: This study suggested that a selected panel of cardiometabolic biomarkers using early-pregnancy random plasma samples predicted GDM comparably to those using mid-pregnancy fasting samples.
Keywords: AUC; Cardiometabolic biomarkers; Clinical accessibility; Decision curve analysis; Gestational diabetes mellitus; Prediction; Pregnancy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol was reviewed and approved by institutional review boards at NICHD and each of the clinical sites. Written informed consent was obtained from all the participants. The IRB approval number is NICHD (09-CH-N152). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47. - PubMed
-
- Assaf-Balut C, García de la Torre N, Durán A, Fuentes M, Bordiú E, Del Valle L, et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): a randomized controlled trial: the St. Carlos GDM prevention study. PLoS ONE. 2017;12:e0185873. - PMC - PubMed
-
- Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity (Silver Spring). 2013;21:904–9. - PubMed
MeSH terms
Substances
Grants and funding
- HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, HHSN275201000001Z/NH/NIH HHS/United States
- HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, HHSN275201000001Z/NH/NIH HHS/United States
- HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, HHSN275201000001Z/NH/NIH HHS/United States
- HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, HHSN275201000001Z/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

