A recurrent ABCC2 c.2439 + 5G > A variant disturbs mRNA splicing and causes Dubin-Johnson syndrome
- PMID: 40682102
- PMCID: PMC12275409
- DOI: 10.1186/s12920-025-02187-4
A recurrent ABCC2 c.2439 + 5G > A variant disturbs mRNA splicing and causes Dubin-Johnson syndrome
Abstract
Background: Hyperbilirubinemia is the main clinical manifestation of Dubin-Johnson syndrome (DJS), of which most cases can be attributed to the variants in the ABCC2 gene. This study aimed to characterize the mechanism of a splicing variant of the ABCC2 gene and interrogate the variant pathogenicity.
Methods: Whole exome sequencing (WES) was performed to identify potential genetic causes. Bioinformatics analysis was performed to predict the variant pathogenicity. Minigene assays were performed to investigate the effects of the identified variant on mRNA splicing. Western blot (WB) experiments were performed to verify the impact of the variant on protein expression. Protein subcellular localization was analyzed by indirect immunofluorescence (IF).
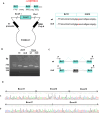
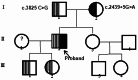
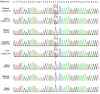
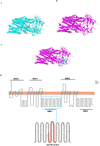
Results: Genetic analysis revealed compound heterozygous variants in ABCC2 gene: the splice-site variant c.2439 + 5G > A inherited from the mother and the nonsense variant c.3825 C > G (p.Y1275X) inherited from the father. The recurrent ABCC2 c.2439 + 5G > A variant can affect mRNA splicing which leads to the skipping of exon 18 and induces the loss of 56 native amino acids, resulting in a shortened MRP2 protein (p.Gly758_Lys813del). The c.2439 + 5G > A variant may cause mislocalization of the mutant protein and significantly reduces its expression.
Conclusion: Our study identifies c.2439 + 5G > A in ABCC2 as a pathogenic variant underlying DJS through aberrant splicing. Critically, the proband's phenotype resulted from compound heterozygous variants leading to biallelic loss of functional protein. These findings highlight the necessity of comprehensive ABCC2 genetic testing for DJS, facilitating accurate diagnosis and personalized patient management.
Keywords: ABCC2; Dubin-johnson syndrome; Heterozygous intronic variant; Minigene assays.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted by the Declaration of Helsinki and approved by the ethics committee of The First Affiliated Hospital of Wenzhou Medical University (No. YS2024-268). Informed consent to participate was obtained from all of the participants and parents of the minor participant in the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Strassburg CP. Hyperbilirubinemia syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and rotor syndrome). Best Pract Res Clin Gastroenterol. 2010;24(5):555–71. - PubMed
-
- Dubin IN, Johnson FB. Chronic idiopathic jaundice with unidentified pigment in liver cells; a new clinicopathologic entity with a report of 12 cases. Med (Baltim). 1954;33(3):155–97. - PubMed
-
- Paulusma CC, et al. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology. 1997;25(6):1539–42. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

