The gut-heart axis: a correlation between Paneth cells' dysfunction, microbiome dysbiosis, and cardiovascular diseases
- PMID: 40682104
- PMCID: PMC12273261
- DOI: 10.1186/s12964-025-02335-4
The gut-heart axis: a correlation between Paneth cells' dysfunction, microbiome dysbiosis, and cardiovascular diseases
Abstract
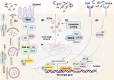
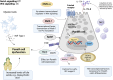
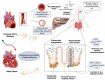
Gut microbiota dysbiosis is characterized by an imbalance in the core microbial equilibrium, leading to changes in the homeostasis of the gastrointestinal tract (GIT) environment. As guardians of the gut microbiota, Paneth cells (PCs) secrete antimicrobial peptides (AMPs) and play a crucial role in maintaining gut integrity and innate immunity in the small intestine. The gut-heart axis has emerged as a critical mediator in cardiovascular disease (CVD) pathogenesis and has drawn significant attention. In this regard, the reciprocal relationship between gut dysbiosis and PC dysfunction has been proposed, which may contribute to a compromised gut barrier and increased systemic inflammation, one of the main drivers of CVD development. It is also well-established that dysfunctional PCs disrupt gut homeostasis and subsequently permit the translocation of pro-inflammatory metabolites like trimethylamine N-oxide (TMAO) while reducing protective short-chain fatty acids (SCFAs), which correlates with atherosclerosis, hypertension, and heart failure.
A better understanding of the underlying mechanisms linking gut health, PCs function, and cardiovascular outcomes is warranted for developing novel gut-target therapies against major CVD risks. This review aimed to comprehensively discuss the predominant role of PCs in the gut-heart axis, some effective compounds on PC function and AMP modulation, and finally, a possible correlation between PC dysfunction and CVD pathogenesis, encouraging future research to further elucidate this crosstalk.
Keywords: Bioinformatics analysis; Cardiovascular disease; Gut barrier; Gut microbiota; Paneth cells.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: N/A. Consent for publication: N/A. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Microbiota in Gut-Heart Axis: Metabolites and Mechanisms in Cardiovascular Disease.Compr Physiol. 2025 Jun;15(3):e70024. doi: 10.1002/cph4.70024. Compr Physiol. 2025. PMID: 40542540 Free PMC article. Review.
-
Gut-heart axis: cardiac remodeling and heart failure in the context of inflammatory bowel disease and dysbiosis.Am J Physiol Gastrointest Liver Physiol. 2025 Jul 1;329(1):G122-G137. doi: 10.1152/ajpgi.00016.2025. Epub 2025 May 19. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40387516 Free PMC article. Review.
-
Analysis of serum S100A12, soluble advanced glycation end products receptor, and gut microbiome in elderly patients with colorectal cancer.World J Gastrointest Oncol. 2025 Jun 15;17(6):106393. doi: 10.4251/wjgo.v17.i6.106393. World J Gastrointest Oncol. 2025. PMID: 40547164 Free PMC article.
-
Dysbiosis and Neurodegeneration in ALS: Unraveling the Gut-Brain Axis.Neuromolecular Med. 2025 Jul 3;27(1):50. doi: 10.1007/s12017-025-08870-0. Neuromolecular Med. 2025. PMID: 40608189 Review.
-
Gut microbiota dysbiosis -associated obesity and its involvement in cardiovascular diseases and type 2 diabetes. A systematic review.Microvasc Res. 2024 Jan;151:104601. doi: 10.1016/j.mvr.2023.104601. Epub 2023 Sep 9. Microvasc Res. 2024. PMID: 37690507
References
-
- Ferrari AJ, Santomauro DF, Aali A, Abate YH, Abbafati C, Abbastabar H, Abd ElHafeez S, Abdelmasseh M, Abd-Elsalam S, Abdollahi A, et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet. 2024;403:2133–2161. - PMC - PubMed
-
- Mocumbi AO. Cardiovascular health care in Low- and Middle-Income countries. Circulation. 2024;149:557–9. - PubMed
-
- Frioux C, Ansorge R, Özkurt E, Ghassemi Nedjad C, Fritscher J, Quince C, Waszak SM, Hildebrand F. Enterosignatures define common bacterial guilds in the human gut microbiome. Cell Host Microbe. 2023;31:1111–e11251116. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

