SNRPB/CCNB1 axis promotes hepatocellular carcinoma progression and cisplatin resistance through enhancing lipid metabolism reprogramming
- PMID: 40682115
- PMCID: PMC12273286
- DOI: 10.1186/s13046-025-03463-y
SNRPB/CCNB1 axis promotes hepatocellular carcinoma progression and cisplatin resistance through enhancing lipid metabolism reprogramming
Abstract
Background: Hepatocellular carcinoma (HCC) is a major cause of cancer-related mortality globally, significantly impacting worldwide health. Hence, identifying key molecular drivers of HCC progression is crucial for enhancing treatment options and prognostic methods. This study explores the function of Small Nuclear Ribonucleoprotein Polypeptides B and B1 (SNRPB) in HCC, unveiling critical pathways that affect the progression of the disease.
Methods: Utilizing multi-dimensional data that integrates bulk RNA sequencing (bulk RNA-seq), single-cell RNA sequencing (scRNA-seq), and spatial transcriptomics (ST) from HCC patients, we have identified SNRPB as a pivotal gene associated with the spliceosome, playing a central role in both tumor initiation and progression. We also investigated the intricate process by which SNRPB influences cyclin B1 (CCNB1) expression through FOXM1-mediated activation, using a combination of bioinformatics, functional assays, Chromatin Immunoprecipitation (ChIP), and Co-Immunoprecipitation (Co-IP) studies. Complementary in vivo experiments and metabolic assays were conducted to explore the relationship between tumor growth and lipid metabolism further. Additionally, evaluations of cisplatin sensitivity were performed, providing an in-depth analysis of influence of SNRPB on HCC.
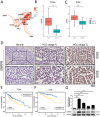
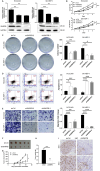
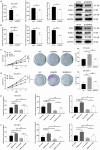
Results: Across multiple cohorts, SNRPB exhibited a marked upregulation within tumors, correlating significantly with poor prognosis. Knockdown of SNRPB suppressed HCC cell proliferation and migration, while promoting apoptosis. Mechanistically, SNRPB regulated CCNB1 expression via FOXM1-mediated transcription, and SNRPB overexpression enhanced lipid metabolism and cisplatin resistance. This increase in drug sensitivity was mediated through alterations in lipid metabolism and the regulatory effects on CCNB1, providing a comprehensive insight into multifaceted role of SNRPB in HCC pathology and potential therapeutic targets. Finally, CCNB1 knockdown reversed the proliferative and tumorigenic effects of SNRPB overexpression in a preclinical HCC model.
Conclusions: SNRPB promoted HCC progression by modulating the FOXM1-CCNB1 axis and lipid metabolism, and could act as a potential therapeutic target to augment chemotherapy sensitivity in HCC.
Keywords: Drug resistance; Hepatocellular carcinoma; Lipid metabolism; SNRPB.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committees of Fudan University Shanghai Cancer Center (No. 050432-4-2108*). The informed consent was obtained from all patients for the archival of their biospecimens and their use in future studies. Competing interests: The authors declare no competing interests.
Figures





References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024.CA Cancer J Clin.2024;74:12–49.10.3322/caac.21820 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

