RBM15 promotes hypoxia/reoxygenation-induced ferroptosis in human cardiomyocytes by mediating m6A modification of ACSL4
- PMID: 40682199
- PMCID: PMC12273425
- DOI: 10.1186/s41065-025-00453-0
RBM15 promotes hypoxia/reoxygenation-induced ferroptosis in human cardiomyocytes by mediating m6A modification of ACSL4
Abstract
Background: Acute myocardial infarction (AMI) refers to the acute necrosis of part of the myocardium caused by persistent and severe myocardial ischemia. The aim of the study was to investigate the effect of RNA binding motif protein 15 (RBM15) and acyl-CoA synthetase long chain family member 4 (ACSL4) on ischemia/reperfusion (I/R)-induced ferroptosis of cardiomyocytes.
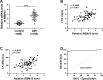
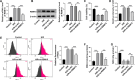
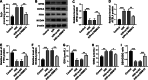
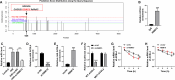
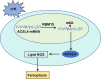
Methods and results: AC16 cells were treated with hypoxia/reoxygenation (H/R) to establish an in vitro myocardial infarction cell model. Quantitative real-time polymerase chain reaction (qRT-PCR) and western blot assay were used to determine gene expression. Cell Counting Kit-8 (CCK-8) assay was conducted to investigate cell viability. Ferroptosis level was evaluated by commercial kits. N6-methyladenosine (m6A) level was examined by M6A quantification analysis. RNA immunoprecipitation (RIP) assay, methylated RNA Immunoprecipitation (meRIP) assay and dual-luciferase reporter assay were adopted to verify the combination between RBM15 and ACSL4. ACSL4 mRNA stability was analyzed by Actinomycin D treatment. RBM15 mRNA level was increased in AMI patients' serums and H/R-induced AC16 cells. Silencing of RBM15 promoted H/R-mediated AC16 cell viability and inhibited H/R-induced AC16 cell oxidative stress and ferroptosis. Moreover, it was demonstrated that RBM15 knockdown inhibited m6A modification of ACSL4 and suppressed the stability of ACSL4 mRNA. Furthermore, ACSL4 overexpression restored the effects of RNM15 silencing on H/R-induced AC16 cell oxidative injury and ferroptosis.
Conclusion: RBM15 silencing repressed H/R-induced ferroptosis in human cardiomyocytes through regulating m6A modification of ACSL4.
Keywords: AC16; ACSL4; AMI; RBM15; m6A.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Written informed consents were obtained from all participants and this study was permitted by the Ethics Committee of Bishan Hospital of Chongqing, Bishan Hospital of Chongqing Medical University. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Teixeira R, Goncalves L, Gersh B. Acute myocardial infarction–historical notes. Int J Cardiol. 2013;167:1825–34. - PubMed
-
- Pollard TJ. The acute myocardial infarction. Prim Care. 2000;27:631–vi649. - PubMed
-
- Rentrop KP, Feit F. Reperfusion therapy for acute myocardial infarction: concepts and controversies from inception to acceptance. Am Heart J. 2015;170:971–80. - PubMed
-
- Burke AP, Virmani R. Pathophysiology of acute myocardial infarction. Med Clin North Am. 2007;91:553–72. ix. - PubMed
-
- Algoet M, Janssens S, Himmelreich U, Gsell W, Pusovnik M, Van den Eynde J, Oosterlinck W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. 2023;33:357–66. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

