Dimerization of Cdc13 is essential for dynamic DNA exchange on telomeric DNA
- PMID: 40683448
- PMCID: PMC12362098
- DOI: 10.1016/j.jbc.2025.110496
Dimerization of Cdc13 is essential for dynamic DNA exchange on telomeric DNA
Abstract
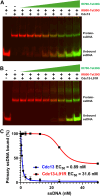
Eukaryotic single-stranded DNA (ssDNA)-binding proteins (ssBPs) protect telomeres from nuclease activity. In Saccharomyces cerevisiae, the ssBP Cdc13 is an essential protein that acts as a central regulator of telomere length homeostasis and chromosome end protection. Cdc13 has high binding affinity for telomeric ssDNA, with a very slow off-rate. Previously, we reported that despite this tight ssDNA binding, Cdc13 rapidly exchanges between bound and unbound telomeric ssDNA substrates, even at sub-stoichiometric concentrations of competitor ssDNA. This dynamic DNA exchange (DDE) is dependent on the presence and length of telomeric repeat sequence ssDNA and requires both Cdc13 DNA binding domains, OB1 and OB3. Here, we investigated if Cdc13 dimerization is important for DDE by characterizing the dimerization mutant Cdc13-L91R. Using mass photometry, we confirmed that Cdc13-L91R fails to dimerize in solution, even in the presence of ssDNA. DDE assays revealed that Cdc13-L91R fails to undergo ssDNA exchange compared to the recombinant wild-type protein. Biolayer interferometry demonstrated that this effect was not due to differences in ssDNA binding kinetics. Thus, dimerization of Cdc13 is essential for DDE, and we model how this may impact telomere biology in vivo.
Keywords: Cdc13; DNA-binding protein; DNA–protein interaction; OB fold; Saccharomyces cerevisiae; dynamic DNA exchange; molecular biology; telomere.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Update of
-
Dimerization of Cdc13 is essential for dynamic DNA exchange on telomeric DNA.bioRxiv [Preprint]. 2025 Mar 26:2025.03.25.645294. doi: 10.1101/2025.03.25.645294. bioRxiv. 2025. Update in: J Biol Chem. 2025 Jul 17;301(8):110496. doi: 10.1016/j.jbc.2025.110496. PMID: 40196551 Free PMC article. Updated. Preprint.
Similar articles
-
Dimerization of Cdc13 is essential for dynamic DNA exchange on telomeric DNA.bioRxiv [Preprint]. 2025 Mar 26:2025.03.25.645294. doi: 10.1101/2025.03.25.645294. bioRxiv. 2025. Update in: J Biol Chem. 2025 Jul 17;301(8):110496. doi: 10.1016/j.jbc.2025.110496. PMID: 40196551 Free PMC article. Updated. Preprint.
-
Cdc13 exhibits dynamic DNA strand exchange in the presence of telomeric DNA.Nucleic Acids Res. 2024 Jun 24;52(11):6317-6332. doi: 10.1093/nar/gkae265. Nucleic Acids Res. 2024. PMID: 38613387 Free PMC article.
-
Comparison of Telomere Structure in Eukaryotes.Arch Razi Inst. 2024 Dec 31;79(6):1365-1374. doi: 10.32592/ARI.2024.79.6.1365. eCollection 2024 Dec. Arch Razi Inst. 2024. PMID: 40606259 Free PMC article. Review.
-
The canonical RPA complex interacts with Est3 to regulate yeast telomerase activity.Proc Natl Acad Sci U S A. 2025 Feb 18;122(7):e2419309122. doi: 10.1073/pnas.2419309122. Epub 2025 Feb 6. Proc Natl Acad Sci U S A. 2025. PMID: 39913192 Free PMC article.
-
Dyskeratosis Congenita and Related Telomere Biology Disorders.2009 Nov 12 [updated 2023 Jan 19]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2009 Nov 12 [updated 2023 Jan 19]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301779 Free Books & Documents. Review.
References
-
- Zakian V.A. In: Saccharomyces Telomeres: Function, Structure, and Replication. Blackburn E.H., Greider C.W., editors. Cold Spring Harbor Laboratory Press; Plainview, NY: 1995. pp. 107–137. Telomeres.
-
- Nugent C.I., Hughes T.R., Lue N.F., Lundblad V. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science. 1996;274:249–252. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

