Activated TBK1 promotes ACSL1-mediated microglia lipid droplet accumulation and neuroinflammation in Parkinson's disease
- PMID: 40684214
- PMCID: PMC12276682
- DOI: 10.1186/s12974-025-03517-0
Activated TBK1 promotes ACSL1-mediated microglia lipid droplet accumulation and neuroinflammation in Parkinson's disease
Abstract
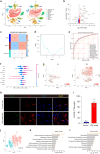
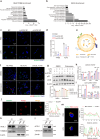
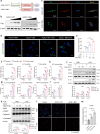
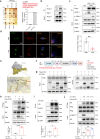
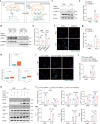
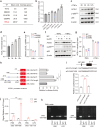
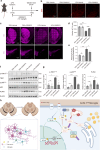
Microglia-mediated neuroinflammation plays a crucial role in the progression of Parkinson’s disease (PD). Dysregulation of lipid droplet homeostasis is a significant factor affecting microglial inflammatory responses, but the mechanisms underlying lipid droplet imbalance in PD are currently unclear. Here, we report a subtype of microglia characterized by high expression of long-chain acyl-CoA synthetase 1 (ACSL1) through single-nucleus RNA sequencing analysis and machine learning algorithms, linking lipid metabolism to PD neuroinflammation. The results of multiple loss- and gain-of-function experiments indicate that ACSL1 localized to the endoplasmic reticulum (ER) promotes lipid droplet accumulation to exacerbate microglial activation and dopaminergic neurons death. Mechanistically, activation of TANK-binding kinase 1 (TBK1) leads to the enrichment of ACSL1 on the endoplasmic reticulum, which generates acyl-CoA that are channelled for lipid droplet biogenesis. Additionally, high expression of ACSL1 promotes activation of TBK1 through Nrdp1-mediated K63 ubiquitination of TBK1, which triggers the amplification of the aforementioned biological effects. Moreover, NF-κB directly binds to the ACSL1 promoter and positively regulates its transcription, resulting in increased ACSL1 expression in microglia. Our findings suggest that manipulating lipid droplet biogenesis by modulating ACSL1 may be a potential strategy for treating neuroinflammation in PD patients.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12974-025-03517-0.
Keywords: ACSL1; Lipid droplet; Microglia; Neuroinflammation; Parkinson’s disease; TBK1.
Conflict of interest statement
Declarations. Ethical approval: All experimental protocols complied with international guidelines and were conducted under the supervision of the Ethics Committee of Beijing Neurosurgical Institute, Capital Medical University (protocol no. BNI202308002). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

