Targeting autophagy and plasminogen activator inhibitor-1 increases survival and remodels the tumor microenvironment in glioblastoma
- PMID: 40684232
- PMCID: PMC12275254
- DOI: 10.1186/s13046-025-03473-w
Targeting autophagy and plasminogen activator inhibitor-1 increases survival and remodels the tumor microenvironment in glioblastoma
Abstract
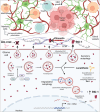
Background: Glioblastoma (GBM), the most common and aggressive type of primary brain tumor, engages multiple survival mechanisms, including autophagy. GBM exploits both degradative and secretory autophagy pathways to support tumor growth and limit the efficacy of standard-of-care treatments. We have previously shown that lucanthone, a blood-brain barrier permeable autophagy inhibitor, reduces tumor burden. However, although lucanthone-treated tumors are significantly smaller in size, they are not completely obliterated, suggesting compensatory survival mechanisms. A critical factor for GBM survival is communication with the tumor microenvironment (TME), which can be programmed by glioma cells to support growth and immunosuppression. Plasminogen activator inhibitor-1 (PAI-1), a secreted serine protease inhibitor, has been implicated in the progression of several cancers, including GBM, and has been shown to be modulated by autophagy in other cancers. The role of PAI-1 in GBM, namely its relationship with intracellular autophagy dysregulation and extracellular TME as a mechanism of tumor survival, remains incompletely understood.
Methods: Murine glioma models were established using intracranial injection of GL261 cells in C57BL/6 mice, followed by autophagy inhibition with intraperitoneal lucanthone and/or PAI-1 inhibition with MDI-2268 chow, and tumors were assessed by immunohistochemistry. In culture, glioma cell lines were challenged with MDI-2268, lucanthone, mitoxantrone, or siRNA-LNPs targeting PAI-1, and assessed by MTT assay, q-RT-PCR, ELISA, invasion assay, immunoblot, and immunocytochemistry. Lysosomal markers and transient transfection with fluorescent vesicular proteins were utilized to evaluate PAI-1 intracellular localization via confocal microscopy. Synergy was analyzed using the HSA model in Combenefit, and statistical analyses included t-tests, ANOVA, and log-rank tests for survival.
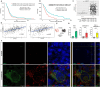
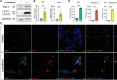
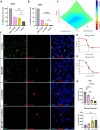
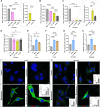
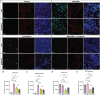
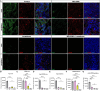
Results: Lucanthone treatment increased intracellular PAI-1 and autophagy markers while reducing active extracellular PAI-1. PAI-1 colocalized with lysosomal markers, suggesting impaired secretory autophagy. PAI-1 inhibition reduced glioma cell viability and invasion. Combination therapy with lucanthone and MDI-2268 drastically decreased tumor volume, prolonged survival, and promoted a pro-inflammatory state in the tumor microenvironment.
Conclusions: Our findings suggest that PAI-1 may be a compensatory survival mechanism in GBM after autophagy inhibition, and that dual targeting of autophagy and PAI-1 disrupts tumor progression and enhances anti-tumor immunity, providing promising evidence for targeting this axis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Obrador E, Moreno-Murciano P, Oriol-Caballo M, López-Blanch R, Pineda B, Gutiérrez-Arroyo JL, Loras A, Gonzalez-Bonet LG, Martinez-Cadenas C, Estrela JM, Marqués-Torrejón MÁ. Glioblastoma therapy: past, present and future. Int J Mol Sci. 2024;25(5):2529. 10.3390/ijms25052529. PMID: 38473776; PMCID: PMC10931797. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

