O-GlcNAcylation of fatty acid synthase is required for its proper subcellular localization, expression level, and activity
- PMID: 40684943
- PMCID: PMC12362114
- DOI: 10.1016/j.jbc.2025.110497
O-GlcNAcylation of fatty acid synthase is required for its proper subcellular localization, expression level, and activity
Abstract
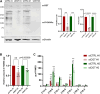
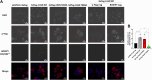
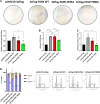
Fatty Acid Synthase (FASN) is involved in various fundamental cellular processes through its pivotal role in producing fatty acids through the de novo lipogenesis pathway. FASN is frequently overexpressed in tumors and participates in cancer cell proliferation. Little has been documented regarding post-translational modifications of FASN. We previously demonstrated that O-GlcNAcylation regulates FASN in mice livers and in the HepG2 hepatic cancer cell line. In the present study, we show that modulation of global O-GlcNAcylation levels impacts fatty acids production in HepG2 cells. We identified serine 595 and threonine 980 as major O-GlcNAcylation sites. While mutation of S595 moderately affects FASN behavior, T980 is crucial for FASN expression, membrane localization, homodimerization, stability, and activity in Hep3B cells. This residue is necessary for FASN properties, promoting cell survival, cell proliferation, and cell cycle progression. Our results suggest that targeting FASN at T980 may open an interesting path for controlling its catalytic activity.
Keywords: O-GlcNAc transferase; O-GlcNAcylation; fatty acid synthase; fatty acids; liver cancer cells.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021;71:209–249. - PubMed
-
- Smith S., Witkowski A., Joshi A.K. Structural and functional organization of the animal fatty acid synthase. Prog. Lipid Res. 2003;42:289–317. - PubMed
-
- Witkowski A., Ghosal A., Joshi A.K., Witkowska H.E., Asturias F.J., Smith S. Head-to-Head coiled arrangement of the subunits of the animal fatty acid synthase. Chem. Biol. décembre 2004;11:1667–1676. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

