Australasian Resuscitation In Sepsis Evaluation: FLUid or vasopressors In emergency Department Sepsis (ARISE FLUIDS) trial: study protocol
- PMID: 40685240
- PMCID: PMC12278162
- DOI: 10.1136/bmjopen-2025-101215
Australasian Resuscitation In Sepsis Evaluation: FLUid or vasopressors In emergency Department Sepsis (ARISE FLUIDS) trial: study protocol
Abstract
Introduction: International consensus guidelines support the initial administration of 30 mL/kg of intravenous fluids for haemodynamic resuscitation of newly diagnosed septic shock. Practice variation exists between the volume of fluids administered and timing of vasopressor commencement. The optimal approach in patients with septic shock is uncertain.
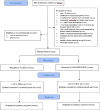
Methods and analysis: Australasian Resuscitation In Sepsis Evaluation: FLUid or vasopressors In emergency Department Sepsis is a 1000-participant multicentre, randomised, open-label, parallel group clinical trial conducted in patients with septic shock presenting to the emergency department in participating sites in Australia, New Zealand and Ireland. Participants are randomised (1:1) to either restricted fluids and early vasopressors or a larger initial intravenous fluid volume and later vasopressors. The primary outcome is days alive and out of hospital at day 90 postrandomisation. Secondary outcomes are all-cause mortality at day 90, time from randomisation until death (to day 90), days alive and at home at day 90 and ventilator-free, vasopressor-free and renal replacement-free days to day 28 postrandomisation and death or disability at 6-month and 12-month postrandomisation. Health-related quality of life will be assessed at day 180 and 12 months following randomisation.
Ethics and dissemination: The study was approved by Northern Sydney Local Health District Human Research Ethics Committee (HREC2020/ETH02874) on 21 January 2021. Patients will be enrolled under a waiver of prior consent. The patient or next-of-kin (or equivalent according to local jurisdiction) is approached at the first available opportunity and given a trial information sheet. According to local approvals, the patient or next-of-kin chooses to either continue in the trial or opt-out/decline continued participation. Results will be disseminated in peer-reviewed journals and presented at academic conferences.
Trial registration number: NCT04569942.
Keywords: Fluid Therapy; Hemodynamics; Sepsis; Shock, Septic; Vasoconstrictor Agents.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Keijzers G, Macdonald SP, Udy AA, et al. The Australasian Resuscitation In Sepsis Evaluation: Fluids or vasopressors in emergency department sepsis (ARISE FLUIDS), a multi‐centre observational study describing current practice in Australia and New Zealand. Emerg Medicine Australasia. 2020;32:586–98. doi: 10.1111/1742-6723.13469. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
