Prunus mume derived extracellular vesicle-like particles alleviate experimental colitis via disrupting NEK7-NLRP3 interaction and inflammasome activation
- PMID: 40685348
- PMCID: PMC12278495
- DOI: 10.1186/s12951-025-03567-9
Prunus mume derived extracellular vesicle-like particles alleviate experimental colitis via disrupting NEK7-NLRP3 interaction and inflammasome activation
Abstract
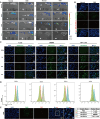
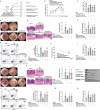
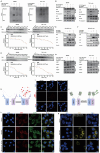
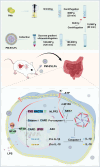
Edible plant derived extracellular vesicle-like particles (EVLPs) have garnered attention as potential therapeutic agents for chronic inflammatory diseases. Prunus mume (PM) is a functional fruit known for its gastrointestinal benefits, yet the detail material basis and potential mechanism remain unclear. Here, we reported that oral administration of prunus mume derived EVLPs (PM-EVLPs) substantially mitigated experimental colitis in mice. The in vivo bio-distribution analysis revealed that PM-EVLPs specifically targeted inflamed colon of colitis mice. Further in vitro studies demonstrated that PM-EVLPs were predominantly internalized by macrophages. The combined treatment with clodronate liposomes confirmed that macrophage was the target cell for PM-EVLPs-mediated anti-colitis activity. Mechanistically, PM-EVLPs selectively inhibited caspase-1 auto-cleavage and IL-1β secretion caused by NLRP3 inflammasome activation, while exerting minimal impact on AIM2, NLRP1 or NLRC4 inflammasome activation. Excluding the effects on mitochondrial ROS generation, K+ efflux or Ca2+ influx, PM-EVLPs disrupted the NEK7-NLRP3 interaction, thereby preventing NLRP3 inflammasome assembly. Notably, the inhibitory activity was attributed to RNAs rather than lipids or proteins within PM-EVLPs. Deep RNA sequencing, coupled with the application of miRNA mimics/inhibitors identified miR159 as the material basis for PM-EVLPs' inhibition of NLRP3 inflammasome activation and anti-colitis efficacy. Collectively, these findings suggest that PM-EVLPs represent a promising nanomedicine with potential as a novel therapeutic strategy for colitis and deserves further investigation and development.
Keywords: NEK7-NLRP3 interaction; NLRP3 inflammasome; PM-EVLPs; Ulcerative colitis; miR159.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The animal experimental protocols were approved by the Experimental Animal Ethics Center of Nanjing University of Chinese Medicine (Approved number: 202012A017; 202103A029; 202207A012; 202304A069). Consent for publication: All the authors agree on the final submission. Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
- No. 81903885/the Program of the National Natural Science Foundation of China
- RDZH2024003/the open foundation of Key Laboratory of Tropical Medicinal Resource Chemistry of Ministry of Education
- TCMADM-2024-05/the Open Project off The Fund of Traditional Chinese Medicine Institute of Anhui Dabie Mountain
- Bengbu Medical University, 2024SYKFD03/the Open Project of Anhui Engineering Technology Research Center of Biochemical Pharmaceutical
LinkOut - more resources
Full Text Sources
Miscellaneous

