Exploring the associations between intraoperative electroencephalographic depression mediated by different anaesthetic drugs and negative postoperative behavioural changes in children undergoing day surgery: a protocol for a two-centre randomised clinical trial
- PMID: 40685366
- PMCID: PMC12278671
- DOI: 10.1186/s13063-025-08962-z
Exploring the associations between intraoperative electroencephalographic depression mediated by different anaesthetic drugs and negative postoperative behavioural changes in children undergoing day surgery: a protocol for a two-centre randomised clinical trial
Abstract
Background: Negative postoperative behavioural changes (NPOBCs) are among the most common complications of paediatric anaesthesia. The association between electroencephalogram (EEG) suppression and postoperative outcomes in previous clinical studies has been limited to delirium occurring early in the anaesthesia recovery room, and there are no reports of associations with negative postoperative behavioural changes in the distant postoperative period. However, this has important implications for children undergoing day surgery who are discharged on the same day after surgery.
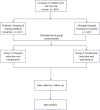
Methods: The objective of this study is to investigate the mediating effects of intraoperative EEG patterns and negative postoperative behavioural changes in children undergoing paediatric day surgery. This study is a two-centre, prospective, randomised, single-blind, controlled trial involving 854 paediatric patients undergoing day surgery at the Children's Hospital of Nanjing Medical University and Zhongda Hospital of Southeast University; these patients are randomly assigned into two groups: the propofol intravenous anaesthesia, which is induced and maintained with propofol, group (Group T) and the sevoflurane inhalation anaesthesia, which is induced and maintained with sevoflurane, group (Group S). The depth of anaesthesia is monitored for both groups of children, and the EEG characteristics of the children are extracted. The primary outcome measure is the incidence of negative behavioural changes during the first three days after surgery. The secondary outcomes include evaluating the incidence of negative behavioural changes at 1, 7, and 28 days postoperatively and investigating the relationship between intraoperative EEG patterns and NPOBCs. The study flow diagram is presented in Fig. 1.
Discussion: The aim of this clinical trial is to prospectively observe the mediating effects of intraoperative EEG forms and negative postoperative behavioural changes in children undergoing paediatric day surgery and to further explore the possible mechanisms of negative postoperative behavioural changes in paediatric anaesthesia. Moreover, in paediatric day surgery, the postoperative discharge assessment of children still lacks the ability to predict negative postoperative behavioural changes, and this study will construct a prediction model of negative postoperative behavioural changes to help anaesthesiologists more comprehensively assess whether children meet the criteria for discharge and to improve the quality of postoperative rehabilitation of children.
Trial registration: The study protocol was registered with the China Clinical Trial Registry ( http://www.chictr.org.cn ) on 1 July 2024 under the registration number ChiCTR2400086403. This trial is retrospectively registered.
Keywords: Anaesthetic protocols; Day surgery; Electroencephalographic forms; Negative postoperative behavioural changes; Paediatric patients.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate {24}: The study protocol and informed consent were approved on 1 April 2024 by the Ethics Committee of the Affiliated Children’s Hospital of Nanjing Medical University (202404014–1) and have since been approved at other centres. Written informed consent will be obtained from participants’ family members or guardians before their participation in the trial. Additionally, informed consent will be sought directly from the children themselves when feasible, particularly for those aged 8 years and older. Consent for publication {32}: All study participants were informed about and consented to the publication of the study results, with the stipulation that no identifying details of the participants would be disclosed. Competing interests {28}: The authors declare that they have no competing interests.
Figures
References
-
- Yuki K, Daaboul DG. Postoperative maladaptive behavioral changes in children. Middle East J Anaesthesiol. 2011;21:183–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources