Nefopam Misuse: A Cross-Sectional Study in France
- PMID: 40685556
- PMCID: PMC12277544
- DOI: 10.1002/ejp.70083
Nefopam Misuse: A Cross-Sectional Study in France
Abstract
Background: Nefopam is a non-opioid analgesic. Nefopam is increasingly prescribed in contraindicated populations, raising questions about the appropriate use of nefopam. This study aimed to evaluate nefopam use in France according to age, epilepsy status and socioeconomic status.
Methods: This retrospective observational study was conducted from January 1, 2016, to December 31, 2022. Data on nefopam use in France were retrieved from the national health insurance information system.
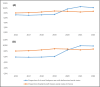
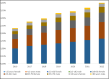
Results: Between 2016 and 2022, 51.8 million boxes of nefopam were dispensed to 6.5 million patients, with a mean age of 56 years and a male-to-female sex ratio of 0.6. Nefopam use increased by 121.7%, whereas codeine use and tramadol use decreased by 7% and 17% respectively, raising the market share of nefopam from 5.0% to 11.6%. Nefopam use increased 2.8-fold in patients under 15 years and 1.7-fold in patients over 65 years, after standardisation. Nefopam use increased 1.7-fold in patients with epilepsy and 1.2-fold in patients with a disadvantaged socioeconomic status. The cost of nefopam was 4.3 times higher than alternative drugs. During the period 2016-2022, 8.23% of the general population received 1 to 4 boxes of nefopam per year, 4.68% received 6 to 19 boxes per year, and 0.91% received ≥ 20 boxes per year.
Conclusions: This study highlights a widespread inappropriate use of nefopam, especially in contraindicated populations and vulnerable groups. The recent availability of the oral form in France may worsen these patterns. Close national and European monitoring is important to assess the extent of such practices and guide future regulation.
Significance statement: This work is a snapshot of nefopam usage in France. It sheds light on contraindicated uses (in subjects under 15 years of age or with epilepsy), not recommended uses (chronic usage, or in the elderly) and reports the usage of this treatment in the most deprived patients. This article cautions against underestimating the dangers and pitfalls of this treatment, especially in light of mounting evidence of pharmacodependence.
© 2025 The Author(s). European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation ‐ EFIC ®.
Figures


References
-
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons . 2009. “Pharmacological Management of Persistent Pain in Older Persons.” Journal of the American Geriatrics Society 57: 1331–1346. - PubMed
-
- Aymard, G. , Warot D., Démolis P., et al. 2003. “Comparative Pharmacokinetics and Pharmacodynamics of Intravenous and Oral Nefopam in Healthy Volunteers.” Pharmacology & Toxicology 92: 279–286. - PubMed
-
- Bannwarth, B. 2004. “Les Antalgiques et Anti‐Inflammatoires Non Stéroïdiens Chez le Sujet Âgé.” Revue du Rhumatisme 71: 534–538.
-
- Capriz, F. , Chapiro S., David L., et al. 2017. “Multidisciplinary Consensus of Experts in Pain and Geriatrics: Use of Analgesics in the Management of Pain in the Elderly (Excluding Anesthesia).” Douleurs: Évaluation–Diagnostic–Traitement 18: 234–247.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

