NAD+-Boosters Improve Mitochondria Quality Control In Parkinson's Disease Models Via Mitochondrial UPR
- PMID: 40685704
- PMCID: PMC12520555
- DOI: 10.1002/advs.202408503
NAD+-Boosters Improve Mitochondria Quality Control In Parkinson's Disease Models Via Mitochondrial UPR
Abstract
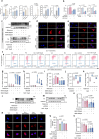
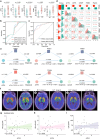
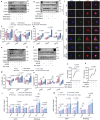
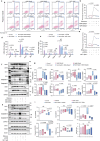
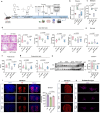
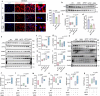
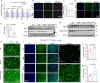
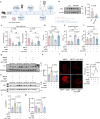
Serving as a pivotal hub for cellular metabolism and intracellular signaling, the mitochondrion has emerged as a crucial organelle whose dysfunction is linked to many human diseases, including neurodegenerative disorders, particularly Parkinson's disease (PD). However, whether mitochondrial quality control (MQC) can be targeted for therapeutic interventions remains uncertain. This study uses clinical samples, molecular biology techniques, pharmacological interventions, and genetic approaches to investigate the significance of NAD+ levels in cross-species models of PD. These results reveal that treatment of rotenone-incubated cells with NAD+ boosters (such as NMN, siCD38, and NAT) increases UPRmt/mitophagy-related MQC, reduces pro-inflammatory cytokine expression, inhibits apoptosis, and strengthen redox reactions. In vivo, NMN supplementation inhibits motor deficit and forestalls the neuropathological phenotypes of MPTP-induced PD mice, which are required for the atf4-related mitochondrial UPR pathway. Notably, bulk omics signatures and metabolomic profiling analyses of the striatum reveal NMN-induced transcriptional changes in genes and proteins involved in mitochondrial homeostasis. Thus, these findings demonstrate that the accelerated pathology in PD models is probably mediated by impaired MQC and that bolstering cellular NAD+ levels alleviates mitochondrial proteotoxic stress and mitigate PD phenotypes.
Keywords: NAD+‐boosters; Parkinson's disease; mitochondria quality control; mitochondrial unfolded protein response; nicotinamide mononucleotide.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bloem B. R., Okun M. S., Klein C., Lancet 2021, 397, 2284. - PubMed
-
- Lawrence G., Holley C. L., Schroder K., Trends Immunol. 2022, 43, 877. - PubMed
-
- Schapira A. H., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D., Lancet 1989, 1, 1269. - PubMed
-
- Moehlman A. T., Youle R. J., Annu. Rev. Cell Dev. Biol. 2020, 36, 265. - PubMed
-
- Schapira A. H., Lancet Neurol. 2008, 7, 97. - PubMed
MeSH terms
Substances
Grants and funding
- LQ23H090007/Natural Science Foundation of Zhejiang Province
- Y19H090059/Natural Science Foundation of Zhejiang Province
- LZ23H090001/Natural Science Foundation of Zhejiang Province
- 2023R01002/Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang
- Y20220164/The Projects of the Wenzhou City Committee of Science and Technology
LinkOut - more resources
Full Text Sources
Medical
