Factors Associated With Self-Report Symptom Screening Adherence in Pediatric Cancer Patients
- PMID: 40686265
- PMCID: PMC12277931
- DOI: 10.1002/cam4.71053
Factors Associated With Self-Report Symptom Screening Adherence in Pediatric Cancer Patients
Abstract
Introduction: Objective was to describe the association between baseline characteristics and the number of Symptom Screening in Pediatrics Tool (SSPedi) assessments completed over an 8-week period.
Methods: This was a sub-analysis of a cluster randomized controlled trial among 10 sites that were randomized to the intervention group. Participants were English- or Spanish-speaking pediatric patients 8-18 years of age newly diagnosed with cancer. Participants were prompted to complete SSPedi three times weekly for 8 weeks. The outcome was the number of SSPedi assessments completed during the 8-week period. Factors associated with the number of assessments were determined using mixed effects Poisson regression.
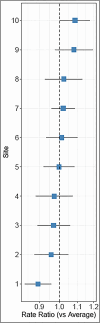
Results: At the 10 intervention sites, 216 patients were included in the analysis. Among these participants, 129 (59.7%) were male, 112 (51.9%) were white, and 83 (38.4%) were Hispanic. The number of SSPedi assessments was significantly higher for participants 11-14 years (rate ratio (RR) 1.13, 95% confidence interval (CI) 1.02-1.25) and 15-18 years (RR 1.15, 95% CI 1.04-1.27) compared to 8-10 years. Participants completed more SSPedi assessments if they were Asian compared to white (RR 1.27, 95% CI 1.10-1.46), non-Hispanic compared to Hispanic (RR 1.15, 95% CI 1.04-1.28) and from families with a household income ≥$60,000 (RR 1.12, 95% CI 1.03-1.21). Participants completed fewer SSPedi assessments if they had solid tumors compared to leukemia (RR 0.91, 95% CI 0.84-0.99).
Conclusion: Adherence to three-times weekly SSPedi varied by age, race, ethnicity, cancer diagnosis, and family income. This information may facilitate interventions to support routine symptom screening in clinical practice.
Trial registration: NCT04614662.
Keywords: adherence; oncology; pediatric; symptom screening.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The following authors declare conflicts of interest: E.O. consults with Jazz Pharmaceuticals.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

