Sleep Deprivation Activates a Conserved Lactate-H3K18la-RORα Axis Driving Neutrophilic Inflammation Across Species
- PMID: 40686333
- PMCID: PMC12520482
- DOI: 10.1002/advs.202504028
Sleep Deprivation Activates a Conserved Lactate-H3K18la-RORα Axis Driving Neutrophilic Inflammation Across Species
Erratum in
-
Correction to "Sleep Deprivation Activates a Conserved Lactate-H3K18la-RORα Axis Driving Neutrophilic Inflammation Across Species".Adv Sci (Weinh). 2025 Nov 6:e21324. doi: 10.1002/advs.202521324. Online ahead of print. Adv Sci (Weinh). 2025. PMID: 41195585 No abstract available.
Abstract
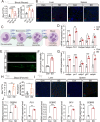
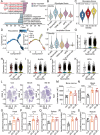
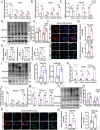
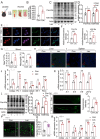
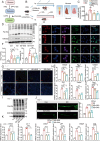
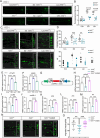
Sleep deprivation critically disrupts physiological homeostasis, impairing development, metabolic balance, and immune regulation, with excessive neutrophil activation being a hallmark consequence. However, the molecular mechanisms underlying sleep deprivation-induced neutrophilic inflammation remain elusive. Here, it is shown that acute sleep deprivation in mice triggers neutrophil hyperactivation, resulting in aberrant peripheral accumulation and a systemic cytokine storm. Mechanistically, this pathology is driven by metabolic dysregulation, specifically, increased glycolytic flux, which elevates tissue lactate levels and enhances histone H3K18 lactylation. Through H3K18 lactylation-specific CUT&Tag profiling, pronounced lactylation enrichment is identified at the promoter of the Rorα gene, directly activating its transcription. Genetic ablation of Rorα or pharmacological inhibition of glycolysis attenuate neutrophil recruitment and mitigated inflammation in sleep-deprived zebrafish. Strikingly, this metabolic‒epigenetic axis is evolutionarily conserved, as demonstrated by the recapitulation of key findings in diurnal zebrafish and pigs. The study reveals a lactate-H3K18 lactylation-Rorα signaling cascade that links sleep deprivation to immune dysregulation, suggesting actionable targets for combating sleep-related inflammatory disorders.
Keywords: Rorα; inflammation; lactylation; neutrophil; sleep deprivation.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
