Novel applications of liquid Biopsy: Comprehensive methodology for circulating biomarker exploration in peripheral blood
- PMID: 40686545
- PMCID: PMC12272590
- DOI: 10.1016/j.jlb.2025.100307
Novel applications of liquid Biopsy: Comprehensive methodology for circulating biomarker exploration in peripheral blood
Abstract
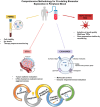
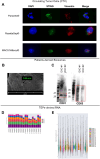
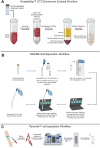
The liquid biopsy (LB) represents a minimally invasive method for cancer screening that has been introduced in clinical practice for over a decade and that can accelerate treatment response assessment. LB allows the analysis of tumor cells or tumor-derived products (e.g. cell-free circulating nucleic acids, extracellular vesicles, and proteins) released from primary or metastatic tumor lesions into blood or other body fluids. In the era of immune-oncology, recent evidence indicates that tumor-specific immune responses can be detected in peripheral immune cells. The improvement of knowledge and the standardization of the isolation methods of these techniques will allow the detection and characterization of circulating tumor and immune biomarkers at an early stage as innovative tools to predict response to therapies. Nowadays, the analysis of peripheral blood mononuclear cells (PBMCs), circulating tumor cells (CTCs), peripheral blood-derived extracellular vesicles (EVs) and circulating tumor RNA (ctRNA) remains under-developed even if these non-invasive techniques can provide the complete genetic landscape of tumors and allow systematic tracking of cancer evolution. In addition, the evaluation of blood circulating cytokines, and early dynamics changes in the PBMCs of patients with solid tumors represent a promising area of research. Here, we present a comprehensive methodological framework for the evaluation of innovative peripheral blood-derived biomarkers. We also address the current challenges in isolation methods and analysis of PBMC, CTC, EVs and TEPs which are crucial for structuring the large amount of comprehensive information obtained from such samples, with the aim of advancing the translational cancer field.
Keywords: Circulating tumor cells; Exosomes; Liquid biopsy; Peripheral blood immune cells; Tumor-educated platelets.
© 2025 The Authors.
Conflict of interest statement
Morgillo F.: receipt of honoraria or consultation fees for speaker, consultancy or advisory roles: Roche, Servier, Incyte, ESMO and MSD. Ciardiello F.: receipt of honoraria or consultation fees for speaker, consultancy or advisory roles: Amgen, Merck KGaA, MSD, Pierre Fabre, Pfizer, Roche and Servier; institutional financial interests, financial support for clinical trials or contracted research: Amgen, Merck KGaA, MSD, Pierre Fabre, Pfizer, Roche and Servier. Servetto A: receipt honoraria or consultation fees for speaker or advisory board from: AstraZeneca, ESMO, MSD, Bristol-Myers Squibb, Gilead, Eli Lilly, Roche, Regeneron, Novartis, Johnson&Johnson, outside the submitted work. Della Corte C.M.: reported receiving personal fees and travel grants from Roche, MSD, Novartis, Lilly, Regeneron, Amgen, Merck, Pfizer and AstraZeneca outside the submitted work. The remaining authors have no conflicts of interest to declare.
Figures



References
-
- De Rosa C., Iommelli F., De Rosa V., Ercolano G., Sodano F., Tuccillo C., Amato L., Tirino V., Ariano A., Cimmino F., di Guida G., Filosa G., di Liello A., Ciardiello D., Martinelli E., Troiani T., Napolitano S., Martini G., Ciardiello F., Papaccio F., Morgillo F., Della Corte C.M. PBMCs as tool for identification of novel immunotherapy biomarkers in lung cancer. Biomedicines. 2024;12(4) - PMC - PubMed
LinkOut - more resources
Full Text Sources

