Effects of empagliflozin on quality of life and healthcare use and costs in chronic kidney disease: a health economic analysis of the EMPA-KIDNEY trial
- PMID: 40686671
- PMCID: PMC12274867
- DOI: 10.1016/j.eclinm.2025.103338
Effects of empagliflozin on quality of life and healthcare use and costs in chronic kidney disease: a health economic analysis of the EMPA-KIDNEY trial
Abstract
Background: Sodium-glucose co-transporter 2 inhibitors (SGLT2i) slow progression of chronic kidney disease (CKD) but there is no randomised evidence of their effects on health-related quality of life (QoL) and healthcare use. We explored the effects of empagliflozin on health-related QoL, healthcare use and UK healthcare costs in the EMPA-KIDNEY trial.
Methods: EMPA-KIDNEY, a randomised, double blind, placebo-controlled, phase 3 trial, was conducted at 241 centres in eight countries (Canada, China, Germany, Italy, Japan, Malaysia, the UK, and the USA), and included participants aged 18 years or older with an estimated glomerular filtration rate (eGFR) of 20 to <45 mL/min/1.73 m2, or with an eGFR of 45 to <90 mL/min/1.73 m2 and a urinary albumin-to-creatinine ratio (uACR) of ≥200 mg/g at screening. They were randomly assigned (1:1) to receive empagliflozin 10 mg once daily or matching placebo. We estimated the effect of empagliflozin (UK£1.31/day) on exploratory outcomes (unless otherwise specified) of quality-adjusted life years (QALYs), UK costs (2023 UK£) of hospital admissions (a prespecified secondary outcome), concomitant medications and end-stage kidney disease (ESKD; a prespecified tertiary outcome) management over 2 years on study treatment (median active-trial follow-up) and on ESKD costs over 2 further years off study treatment (median post-trial follow-up) using shared parameter models analysing outcomes together with time to death or negative binomial models. The trial is registered with ClinicalTrials.gov, NCT03594110.
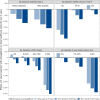
Findings: Between May 15, 2019 and April 16, 2021, 6609 participants were randomly assigned to empagliflozin (3304 participants) or matching placebo (3305 participants) in the active-trial which lasted for a median of 2.0 years. Among them, 4891 (74%) were enrolled in the post-trial follow-up. Per participant allocated to empagliflozin over 2 years, total empagliflozin cost was £826 (95% confidence interval: 818 to 835), QALYs were 0.012 higher (0.001 to 0.022), with less cost for hospital admission (-£239, -449 to -29), concomitant medications (-£130, -214 to -47), and management of ESKD (-£208, -414 to -2) compared to placebo. Over a further 2 years of post-trial follow-up off study treatment, there were additional per participant ESKD cost savings (-£842, -1441 to -242), resulting in net total healthcare cost of -£593 (-1384 to 198) over 4 years. The probability of 2 years of empagliflozin treatment being cost-effective at £20 K threshold in the UK was 43% over 2 years of follow-up and 91% over 4 years. The relative effects of empagliflozin on each cost component were similar across categories by baseline levels of eGFR, uACR and diabetes status, with larger reductions in healthcare costs estimated in categories at higher risk of CKD progression.
Interpretation: In EMPA-KIDNEY, 2 years treatment with empagliflozin improved QALYs, and reduced use and cost of other healthcare, resulting in high likelihood of cost-effectiveness across a broad range of patients with CKD. The study's key limitation is its relatively short active treatment period and follow-up duration, which may lead to underestimation of the cost-effectiveness of long-term SGLT2i treatment in CKD.
Funding: Boehringer Ingelheim, Germany; Eli Lilly, USA; Medical Research Council, UK; British Heart Foundation, UK; Health Data Research, UK; National Institute for Health and Care Research, UK.
Keywords: Chronic kidney disease (CKD); Empagliflozin; Health care costs; Health care use; Quality-adjusted life-year (QALY).
© 2025 The Author(s).
Conflict of interest statement
The EMPA-KIDNEY trial was initiated, designed, conducted, analysed and reported by the University of Oxford with a Steering Committee of experts. This paper has not been published previously in whole or part. The Clinical Trial Service Unit and Epidemiological Studies Unit (Oxford, UK) has a staff policy of not accepting honorarium or other payments from the pharmaceutical industry, except for the reimbursement of costs to participate in scientific meetings (see https://www.ctsu.ox.ac.uk/about/ctsu_honoraria_25june14-1.pdf). JZ, CW, NS, PKJ, KJM, NA, RA, DP, ChW, MJL, CB, RH, WGH, BM report grant funding paid to their institution (the University of Oxford) from Boehringer Ingelheim and Eli Lilly, and funding from the United Kingdom Medical Research Council (MRC) (to the Clinical Trial Service Unit and Epidemiological Studies Unit; reference no., MC_UU_00017/3), the British Heart Foundation, National Institute for Health and Care Research Biomedical Research Council, and Health Data Research (UK). Additionally: NS reports institutional grant funding from Novo Nordisk; and leadership role in Nephrology Dialysis and Transplant journal. PKJ reports institutional grant funding from Novartis. DP additionally reports institutional grant funding from Novo Nordisk. ChW additionally reports consultancy fees or honoraria for lectures as part of his affiliation with Wurzburg from VeraTX, Alexion, MSDBayer, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, CSLVifor, Lilly, NovoNordisk; board membership of AKTN – Australasian Kidney Trials network; and a leadership role in European Renal Association. MJL additionally reports institutional grant funding from Novartis, Janssen, GV, Flu Lab, Schmidt Future, NHS England, Wellcome, Bill & Melinda Gates Foundation; research contract with Sanofi, Regeneron, Moderna, BioNTech, Apollo Therapeutics, Verve Therapeutics and GSK; support to attend meetings from Boehringer Ingelheim; unpaid advisory role to European Society of Cardiology. CB additionally reports institutional grant funding from NIHR HTA and Health Data Research UK; participation on a Data Safety Monitoring Board or Advisory Board related to Merck, NIHR HTA, the British Heart Foundation; and leadership roles as European Society of Cardiology Chair of Committee on Practice Guidelines and with NIHR HTA (Chair: ATTACK: Aspirin To Target Arterial Events in Chronic Kidney Disease; & DASH: Desmopressin for acute stroke due to haemorrhage). RH additionally reports participation on a Data Safety Monitoring Board or Advisory Board related to Lilly (no payments received). WGH additionally reports personal fellowship grant from Kidney Research UK. BM additionally reports institutional grant funding from NIHR (HTA, Barts Biomedical Research Centre); support to attend meetings from the European Society of Cardiology and Kidney Disease Improving Global Outcomes; and unpaid role on the ESC Clinical Practice Guidelines Committee. JBG reports institutional grant funding from Merck, Roche, Boehringer Ingelheim, Lilly, Bluedrop; and consulting fees from AstraZeneca, Corcept, NovoNordisk, Bayer, Boehringer Ingelheim, Valo, Lilly, Vertex, Mineralys. DZIC has received honoraria from Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, Abbvie, Janssen, AMGEN, Bayer, Prometic, BMS, Maze, Gilead, CSL-Behring, Otsuka, Novartis, Youngene, Lexicon, Inversago, GSK, Biobridge, Vantage, Altimmune and Novo-Nordisk and has received operational funding for clinical trials from Boehringer Ingelheim-Lilly, Merck, Janssen, Sanofi, AstraZeneca, CSL-Behring and Novo-Nordisk, Bayer. KRT reports grant funding from the National Institutes of Health, NIH (NIDDK, NHLBI, NCATS, NIMHD), the Centers for Disease Control and Prevention (CDC), Travere, Bayer and Doris Duke Foundation; consulting fees from Lilly, Boehringer Ingelheim, Astra Zeneca, Novo Nordisk, Bayer and ProKidney; honoraria from Astra Zeneca, Novo Nordisk and Bayer; support to attend meetings from Novo Nordisk; support for role on Data Safety Monitoring Board from AstraZeneca; unpaid roles on Data Safety Monitoring/Advisory Boards for NIDDK and George Clinical; paid roles on Data Safety Monitoring Board for AstraZeneca; and unpaid leadership roles as Chair, Diabetic Kidney Disease Collaborative Taskforce, American Society of Nephrology and Board of Directors, Kidney Disease Improving Global Outcomes, and Controversies Conference on Kidney Disease Prevention, and Chair, Program Committee for Kidney Week 2025, American Society of Nephrology. JL and PC declare no competing interests.
Figures


References
-
- Nuffield Department of Population Health Renal Studies Group SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–1801. - PMC - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105(4S):S117–S314. - PubMed
-
- National Institute for Health and Care Excellence Dapagliflozin for treating chronic kidney disease. 2022. www.nice.org.uk/guidance/ta775 - PubMed
-
- National Institute for Health and Care Excellence Empagliflozin for treating chronic kidney disease. 2023. www.nice.org.uk/guidance/ta942 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

