Oxidation Reactions Promoted or Catalyzed by Organotellurium Compounds
- PMID: 40687001
- PMCID: PMC12268469
- DOI: 10.1021/acsomega.5c03157
Oxidation Reactions Promoted or Catalyzed by Organotellurium Compounds
Abstract
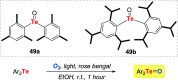
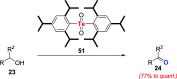
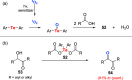
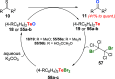
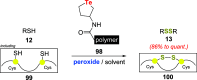
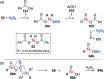
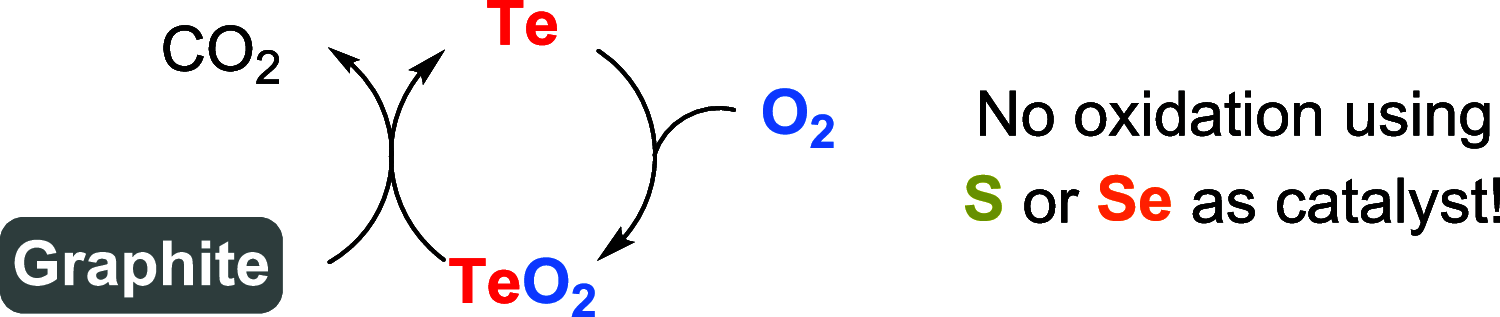
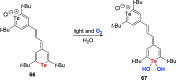
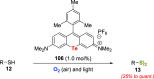
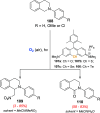
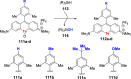
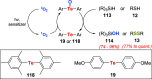
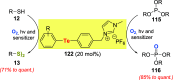
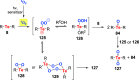
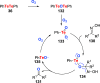
Oxidation of organic substrates is a pivotal transformation, with profound chemical and biological implications. This review covers the synthetic application of organotellurium compounds as promoters or activators of oxidizing agents in such reactions. This research field has evolved from utilizing stoichiometric amounts of organotellurium as oxidizing agents, producing copious amounts of side products, to using this class of compounds as catalysts. Another unique feature associated with using organotellurium compounds as catalysts for oxidation is the possibility of employing less hazardous oxidizing agents, such as peroxides or combinations of oxygen, photosensitizers, and light. These characteristics render the application of organotellurium compounds attractive from both an economic and environmental perspective.
© 2025 The Authors. Published by American Chemical Society.
Figures
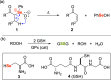


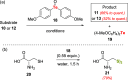
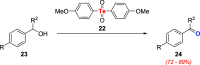
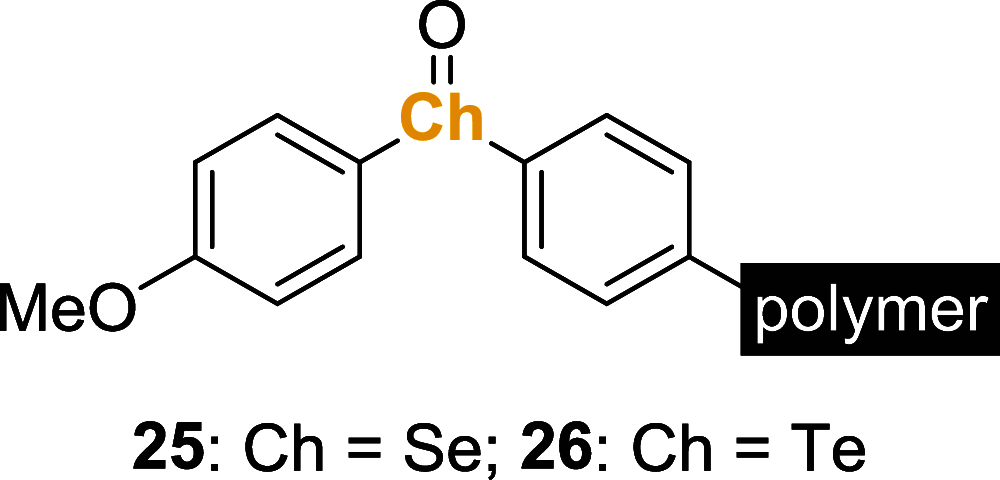
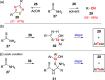
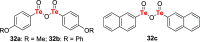
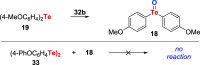
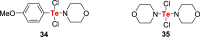
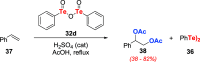
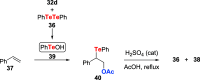
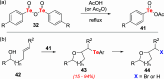
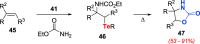


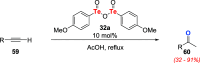
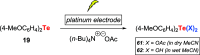
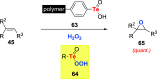

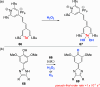

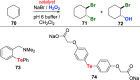
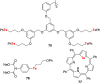
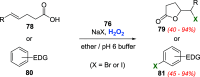
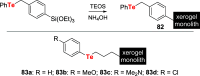
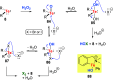
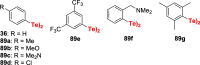

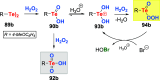
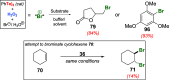

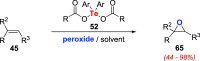




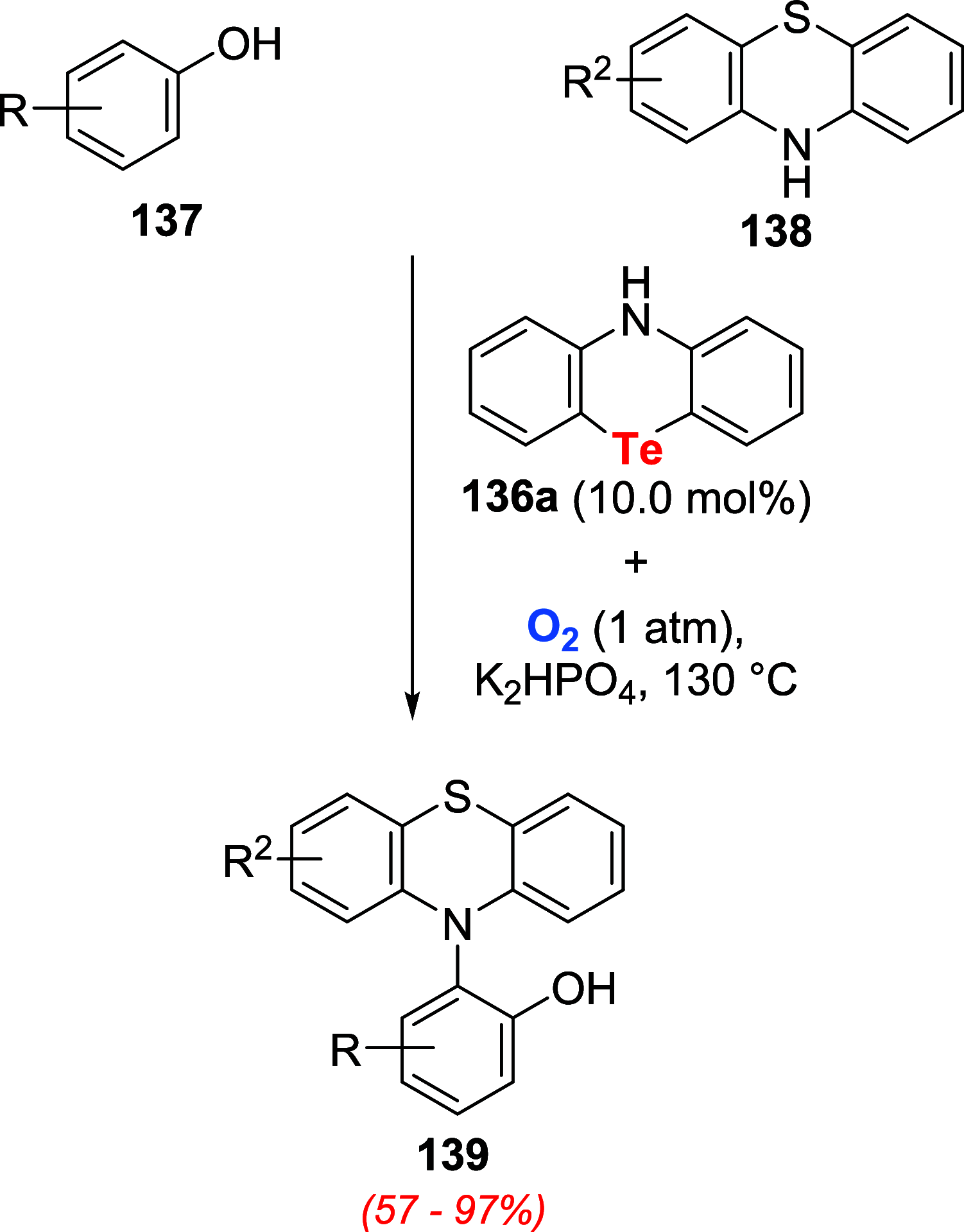
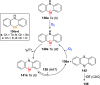
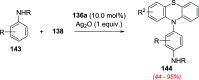
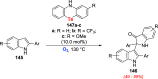
References
-
- Riley, H. L. British Patent. BP354798 1930.
-
- Riley H. L., Friend N. A. C.. 335. Selenium dioxide, a new oxidising agent. Part II. Its reaction with some unsaturated hydrocarbons. J. Chem. Soc. 1932:2342–2344. doi: 10.1039/jr9320002342. - DOI
-
- Waitkins G. R., Clark C. W.. Selenium Dioxide: Preparation, Properties, and Use as Oxidizing Agent. Chem. Rev. 1945;36:235–289. doi: 10.1021/cr60115a001. - DOI
-
- Reich H. J., Reich I. L., Renga J. M.. Organoselenium chemistry a-Phenylseleno carbonyl compounds as precursors for a,b-unsaturated ketones and esters. J. Am. Chem. Soc. 1973;95:5813–5815. doi: 10.1021/ja00798a090. - DOI
-
- Sharpless K. B., Lauer R. F., Teranishi A. Y.. Electrophilic and nucleophilic organoselenium reagents. New routes to a,b-unsaturated carbonyl compounds. J. Am. Chem. Soc. 1973;95:6137–6139. doi: 10.1021/ja00799a062. - DOI
Publication types
LinkOut - more resources
Full Text Sources
