Simulation-Guided Engineering of Mg-Doped Silica Membranes via DFT and MATLAB: Toward High-Efficiency Desalination and Antibacterial Protection
- PMID: 40687046
- PMCID: PMC12268401
- DOI: 10.1021/acsomega.5c04165
Simulation-Guided Engineering of Mg-Doped Silica Membranes via DFT and MATLAB: Toward High-Efficiency Desalination and Antibacterial Protection
Abstract
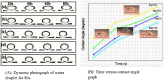
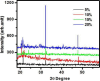
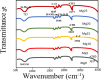
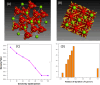
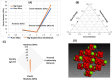
In this work, magnesium (Mg)-doped silica membranes were fabricated via a facile sol-gel method and systematically evaluated for multifunctional water purification applications with a specific focus on membrane distillation (MD). In this study, controlled incorporation of magnesium into the silica network effectively improves the membrane's hydrophobicity, structural compactness, and functional performance. This enhancement presents a novel and efficient strategy for developing high-performance membranes tailored to water desalination applications. Characterization using FTIR and SEM confirmed successful integration of Mg and trimethoxy-octyl-silane (C8TMOS) into the silica matrix, leading to the formation of a dense, uniform surface with tunable hydrophobicity. Water contact angle measurements revealed superhydrophobic behavior at higher Mg loadings, ranging from 80° to 140°, indicating reduced wettability and improved liquid entry pressure. DCMD experiments using NaCl and Na2SO4 solutions (1000 ppm) showed significant enhancement in salt rejectionup to 98.3%and a stable permeate flux of 32 L/m2·h, especially for membranes doped with 15 wt % Mg. These improvements are attributed to densification and surface modification induced by Mg cross-linking, which inhibited pore wetting and maintained membrane stability during long-term operation. EDX and XRD confirmed elemental distribution and the amorphous structure of the membranes, respectively. Furthermore, density functional theory (DFT) simulations provided insight into the role of Mg in improving electronic structure, ion repulsion, and mechanical robustness at the molecular level. The membranes also exhibited strong antibacterial efficacy against Staphylococcus aureus and Klebsiella pneumoniae, suppressing over 90% bacterial regrowth. This multi-functionality, combining desalination efficiency, antifouling resistance, and antimicrobial activity, makes Mg-doped silica membranes a promising and scalable solution for sustainable water treatment, including high-salinity brines and industrial effluents.
© 2025 The Authors. Published by American Chemical Society.
Figures





References
-
- Nayak M. C., Isloor A. M., Lakshmi B., Marwani H. M., Khan I.. Polyphenylsulfone/multiwalled carbon nanotubes mixed ultrafiltration membranes: Fabrication, characterization and removal of heavy metals Pb2+, Hg2+, and Cd2+ from aqueous solutions. Arabian J. Chem. 2020;13(3):4661–4672. doi: 10.1016/j.arabjc.2019.10.007. - DOI
-
- Zhang H., He D., Niu S., Qi H. T.. Tuning the microstructure of organosilica membranes with improved gas permselectivity via the co-polymerization of 1, 2-bis (triethoxysilyl) ethane and 1, 2-bis (triethoxysilyl) methane. Int. J. Hydrogen Energy. 2021;46(33):17221–17230. doi: 10.1016/j.ijhydene.2021.02.139. - DOI
LinkOut - more resources
Full Text Sources
