Synthesis and X‑ray Structures of Bis-Functional Resorcinarene Crown Ethers
- PMID: 40687269
- PMCID: PMC12272548
- DOI: 10.1021/acs.cgd.5c00295
Synthesis and X‑ray Structures of Bis-Functional Resorcinarene Crown Ethers
Abstract
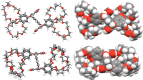
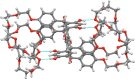
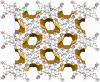
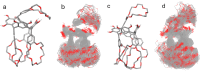
Two resorcin[4]-arene-crowns were synthesized with crown ether substituents as the lower rim functionalities, with either the Crown-6 (TRC6) or Crown-7 (TRC7) moieties being incorporated. X-ray crystallographic data show that both molecules prefer the boat conformation in the solid state. The crown ethers were observed in an askew anti-arrangement in TRC6 and a syn-arrangement in TRC7. TRC6 crystallized with four DMF and four H2O molecules. Two of the DMF solvent molecules are hydrogen bonded to the resorcinolic OHs, and the other two are entrapped as a dimer between two ether moieties from adjacent TRC6 molecules. In TRC7, four intramolecular hydrogen bonds from the same resorcinol OH group to the crown ether oxygens are observed, with O···O contact distances of 2.742(2) Å and 2.872(2) Å and O-H···O angles of 169.8° and 175.4°. Host-guest binding of potassium and rubidium cations as their chloride and tetrafluoroborate salts in highly competitive DMSO shows 1:1 and 1:2 host-guest complexes for TRC6 and TRC7, respectively. More intense NMR signal changes were observed in less competitive acetonitrile solvent with the tetrafluoroborate salts.
© 2025 The Authors. Published by American Chemical Society.
Figures





References
-
- Twum K., Nadimi S., Osei F. B., Puttreddy R., Ojong Y. B., Hayward J. J., Rissanen K., Trant J. F., Beyeh N. K.. The ‘Nitrogen Effect’: Complexation with macrocycles potentiates fused heterocycles to form halogen bonds in competitive solvents. Chem. - Asian J. 2023;18:e202201308. doi: 10.1002/asia.202201308. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
