Substrate stiffness and pressure alter retinal Müller glia response and extracellular matrix production
- PMID: 40687493
- PMCID: PMC12275231
- DOI: 10.1016/j.bbiosy.2025.100114
Substrate stiffness and pressure alter retinal Müller glia response and extracellular matrix production
Abstract
Background: The retina is highly influenced by its mechanical environment, with Müller glia (MG) acting as key mechanosensors and extracellular matrix (ECM) producers. This study examined MG responses to substrate stiffness and high pressure (HP), and whether TGF-β1 modulation could mitigate these effects.
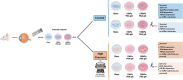
Methods: Primary MG from adult rat retinas were cultured on glass (Young's modulus E'=∼1 gigapascal (GPa)) and polyacrylamide gels (10 kPa and 100 kPa). MG were exposed to atmospheric and 70 mmHg (HP) conditions, with TGF-β1 pharmacologically blocked.
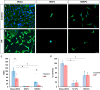
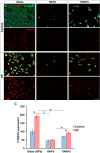
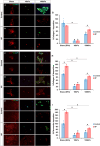
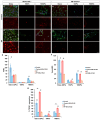
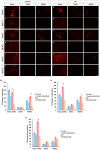
Results: On glass and 100 kPa gels, MG survival, cell area, and ECM deposition (collagen I, IV, and fibronectin) increased, with cells adopting a fusiform shape and more dedifferentiated state. Under HP, survival decreased on stiffer substrates, though cell area and morphology remained unchanged. HP increased ECM deposition, which was reduced by TGF-β1 inhibition.
Conclusions: Our findings suggest that MG response to mechanical stress alter their survival and cell area, and increases ECM secretion, highlighting TGF-β1 as a potential therapeutic target.
Keywords: Extracellular matrix; Müller glia; Polyacrylamide gels; Pressure; Stiffness; TGF-β1.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Franze K., Francke M., Günter K., Christ A.F., Körber N., Reichenbach A., et al. Spatial mapping of the mechanical properties of the living retina using scanning force microscopy. Soft Matter. 2011;7:3147.
-
- Vecino E., Kwok J.C.F. IntechOpen; 2016. The extracellular matrix in the nervous system: the good and the bad aspects.
-
- Reinhard J., Joachim S.C., Faissner A. Extracellular matrix remodeling during retinal development. Exp Eye Res. 2015;133:132–140. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
