Establishment of a clinically relevant beagle model for periprosthetic joint infection with 3D-printed prostheses and multimodal evaluation
- PMID: 40687554
- PMCID: PMC12270800
- DOI: 10.1016/j.jot.2025.05.007
Establishment of a clinically relevant beagle model for periprosthetic joint infection with 3D-printed prostheses and multimodal evaluation
Abstract
Objective: Periprosthetic joint infection (PJI) poses significant challenges to arthroplasty outcomes, necessitating translational animal models for pathogenesis studies and therapeutic development. This study aimed to establish a standardized Beagle PJI model by integrating species-specific 3D-printed femoral prostheses with quantitative bacterial inoculation, while evaluating the dose-dependent effects of Staphylococcus aureus (S. aureus) on infection progression.
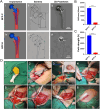
Methods: Two titanium alloy prostheses were designed using CT-based anatomical data: BFP-C (canine-optimized) and BFP-H (human-derived). Prostheses underwent mechanical compression tests, finite element analysis (FEA) simulating postoperative and osseointegration phases, and in vivo validation in Beagles. The optimized BFP-C was selected for PJI model construction via hemi-hip arthroplasty (HHA), with intraoperative inoculation of S. aureus ranging from 250 to 10^8 colony-forming units (CFU). Longitudinal evaluation included radiography (X-ray/CT), mechanical pull-out tests, histopathology (H&E/Masson/Giemsa staining), bacterial cultures, and mobility assessments using open-field behavioural tracking.
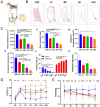
Results: BFP-C exhibited superior biomechanical compatibility, with 12.3-fold higher yield strength (6836 ± 157 N vs. 553 ± 49 N) and 97 % reduction in bone strain (0.71 % vs. 20.32 %) compared to BFP-H. All inoculated groups developed PJI with dose-dependent severity: ultra-high-dose (10^8 CFU) groups displayed severe osteolysis (pull-out strength: 24 ± 8 N vs. 924 ± 45 N in controls), biofilm formation, and mobility impairment (74 % reduction in distance travelled, 2003 ± 276 cm vs. 7976 ± 333 cm in controls), whereas low-dose (250 CFU) groups established PJI with milder manifestations, evidenced by sinus tract formation, 55.1 % reduction in pull-out strength (406 ± 15 N vs. 924 ± 45 N in controls), and concordant radiological/histopathological signs of infection. Imaging examinations revealed differential osteolytic patterns corresponding to bacterial loads. Combined wound evaluation and microbiological analyses confirmed consistent infection establishment across all replicates.
Conclusion: This Beagle PJI model successfully recapitulates clinical infection dynamics, emphasizing the critical role of species-specific prosthesis design and standardized bacterial quantification. The integrated multimodal evaluation system (imaging, biomechanical, and behavioural analyses) demonstrated both the reliability of the model and its sensitivity in detecting infection progression. Its modular design supports customization for studying biofilm-resistant implants or antibiotic delivery systems. These findings not only provide a critical tool for mechanistic PJI research but also establish a theoretical foundation for clinical translation, with the quantitative multimodal framework directly informing diagnostic and therapeutic strategies.
Translational potential: Beyond serving as a preclinical platform for anti-infective therapies, the model provides actionable insights into optimizing human prosthetic biomechanics, such as reducing stress shielding through FEA-informed design principles. The 3D printing workflow further demonstrates rapid prototyping capabilities for patient-specific orthopaedic implants.
Keywords: 3D printed prosthesis; Beagle model; Periprosthetic joint infection (PJI); Translational biomechanics.
© 2025 The Authors.
Conflict of interest statement
All the authors declare no conflicts of interest with the contents of this article.
Figures






Similar articles
-
Is 18 F-fluoride PET/CT an Accurate Tool to Diagnose Loosening After Total Joint Arthroplasty?Clin Orthop Relat Res. 2025 Mar 1;483(3):415-428. doi: 10.1097/CORR.0000000000003228. Epub 2024 Sep 11. Clin Orthop Relat Res. 2025. PMID: 39293088
-
How Often Does Bacteremia Occur in Patients With Chronic Periprosthetic Joint Infection? A Prospective, Observational Study.Clin Orthop Relat Res. 2025 Jan 21;483(7):1206-1214. doi: 10.1097/CORR.0000000000003367. Clin Orthop Relat Res. 2025. PMID: 39843348
-
Bacteriophage therapy as an innovative strategy for the treatment of Periprosthetic Joint Infection: a systematic review.Int Orthop. 2024 Nov;48(11):2809-2825. doi: 10.1007/s00264-024-06295-1. Epub 2024 Sep 10. Int Orthop. 2024. PMID: 39254722 Free PMC article.
-
What Are the Functional, Radiographic, and Survivorship Outcomes of a Modified Cup-cage Technique for Pelvic Discontinuity?Clin Orthop Relat Res. 2024 Dec 1;482(12):2149-2160. doi: 10.1097/CORR.0000000000003186. Epub 2024 Jul 9. Clin Orthop Relat Res. 2024. PMID: 38991223
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
Cited by
-
Editorial: From molecular insights to innovative implants in degenerative skeletal disorders.J Orthop Translat. 2025 Jul 30;53:A1-A2. doi: 10.1016/j.jot.2025.07.009. eCollection 2025 Jul. J Orthop Translat. 2025. PMID: 40933228 Free PMC article. No abstract available.
References
-
- Kuehling T., Schilling P., Bernstein A., Mayr H.O., Serr A., Wittmer A., et al. A human bone infection organ model for biomaterial research. Acta Biomater. 2022;144:230–241. - PubMed
-
- Trampuz A., Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep. 2008;10(5):394–403. - PubMed
-
- Sadhwani S., Kamson A., Frear A.J., Sadaka N., Urish K.L. Current concepts on the clinical and economic impact of periprosthetic joint infections. Orthop Clin N Am. 2024;55(2):151–159. - PubMed
LinkOut - more resources
Full Text Sources

