Neuronal synchronization in Dr oso phila
- PMID: 40687819
- PMCID: PMC12275062
- DOI: 10.1016/j.isci.2025.112942
Neuronal synchronization in Dr oso phila
Abstract
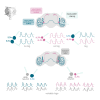
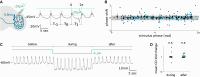
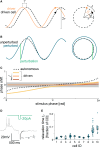
Collective rhythms are intrinsic to biological processes across temporal and spatial scales. In the brain, synchronized neuronal oscillations underlie collective rhythms essential for complex functions. Neuronal oscillations were reported in individual Drosophila neurons that underlie circadian and sleep behaviors. However, it is still unclear whether and how these participate in a collective rhythm. We perform whole-cell patch clamp recordings and demonstrate that membrane potential oscillations disappear after blocking nicotinic acetylcholine receptors. Perturbations to membrane potential do not change the phase of oscillation, further suggesting they depend on external inputs. We propose a theoretical description that accounts for experimental observations and predicts phase-locked neuronal synchronization. Simultaneous electrophysiological recordings of neuronal pairs confirm this prediction and show that this is a widespread phenomenon in accessory medulla neurons. Our findings suggest the possibility that brain waves may arise from collective neuronal activity within this region of the fly brain.
Keywords: Neuroscience; Sensory neuroscience.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






References
-
- Winfree A.T. Springer-Verlag; 1980. The Geometry of Biological Time.
-
- Ender P., Gagliardi P.A., Dobrzyński M., Frismantiene A., Dessauges C., Höhener T., Jacques M.-A., Cohen A.R., Pertz O. Spatiotemporal control of erk pulse frequency coordinates fate decisions during mammary acinar morphogenesis. Dev. Cell. 2022;57:2153–2167.e6. - PubMed
-
- Christoph J., Chebbok M., Richter C., Schröder-Schetelig J., Bittihn P., Stein S., Uzelac I., Fenton F.H., Hasenfuß G., Jr., Luther S., Gilmour R.F., Jr. Electromechanical vortex filaments during cardiac fibrillation. Nature. 2018;555:667–672. - PubMed
-
- Marder E., Bucher D. Central pattern generators and the control of rhythmic movements. Curr. Biol. 2001;11:R986–R996. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

