A Phase II Study of Cabozantinib in Patients With MET-Altered Lung Cancers
- PMID: 40687931
- PMCID: PMC12271782
- DOI: 10.1016/j.jtocrr.2025.100857
A Phase II Study of Cabozantinib in Patients With MET-Altered Lung Cancers
Abstract
Introduction: Only type I MET tyrosine kinase inhibitors (TKIs) are approved for treating MET-altered NSCLCs. Preclinically, type II TKIs, such as cabozantinib, can rescue progression on type I TKIs. This phase 2 trial (NCT01639508) evaluated the activity of cabozantinib in patients with MET-dependent lung cancers, including TKI-pretreated cancers.
Methods: This phase 2 trial with a Simon two-stage minimax design treated patients with metastatic, MET-altered lung cancers with cabozantinib (60 mg daily) until progression or intolerable toxicity. The primary end point was objective response rate (ORR). We prespecified that cabozantinib would be considered a useful agent if at least a 20% ORR was observed. Secondary end points included progression-free survival, overall survival, and safety.
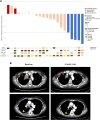
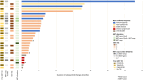
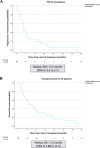
Results: We enrolled 28 patients, 23 patients (82%) with only a MET exon 14 alteration, two patients (7%) with MET amplification, and three patients (11%) with concurrent MET exon 14 alteration and amplification. There were 24 patients (86%) previously treated with a type I MET TKI. The ORR was 20% (5/25 assessable patients; 95% confidence interval [CI]: 8.9%-39.1%), with five partial responses (duration ranged from 4 to 39 mo). Four of five responders were type I MET TKI pretreated. The median progression-free survival and overall survival were 4.5 (95% CI: 3.3-5.7) months and 7.2 (95% CI: 2.9-11.5) months, respectively. Dose modification and discontinuation occurred in 64% (18/28) and 7% (2/28) of patients, respectively.
Conclusion: This trial met its primary end point. Importantly, we demonstrated that cabozantinib, a type II MET TKI, could benefit patients with MET-altered lung cancers previously treated with type I MET TKIs.
Keywords: Cabozantinib; Clinical trial; MET alterations; Non–small cell lung cancer.
© 2025 The Authors.
Conflict of interest statement
Dr. Harada reports receiving honoraria/serving on the advisory boards of AstraZeneca, BeiGene, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Johnson & Johnson, Merck, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda. Dr. Kris reports receiving honoraria/serving on the advisory boards of AstraZeneca, Bristol Myers Squibb, and Daiichi Sankyo; serving on the Data and Safety Monitoring Board of Merck; receiving editorial support from AstraZeneca and Genentech; and receiving Continuing Medical Education honoraria from Medscape, OncLive, Physicians Education Resources, Targeted Oncology, WebMD, and MJH Life Sciences. Dr. Drilon reports receiving honoraria/serving on the advisory boards of Ignyta, Genentech, Roche, MORE Health, AXIS, Loxo, Eli Lilly and Company, Bayer, AbbVie, EPG Health, Takeda, Ariad, Millenium, 14ner, Elevation Oncology, Harborside Nexus, TP Therapeutics, ArcherDX, Liberum, AstraZeneca, Monopteros, RV More, Pfizer, Novartis, Ology, Blueprint Medicines, EMD Serono, Amgen, Helsinn, Medendi, TouchIME, BeiGene, Repare RX, Janssen, BergenBio, Nuvalent, Entos, Hengrui Therapeutics, Merus, Treeline Bio, Exelixis, Chugai Pharmaceutical, Prelude, Tyra Biosciences, Remedica Ltd., Applied Pharmaceutical Science, Inc., Verastem, mBrace, Treeline, MonteRosa, AXIS, and EcoR1; having associated research paid to institution by Pfizer, Exelixis, GlaxoSmithKline, Teva, Taiho, and PharmaMar; receiving royalties from Wolters Kluwer; receiving other (food/beverage) support from Merck, Puma, Merus, and Boehringer Ingelheim; and receiving Continuing Medical Education honoraria from Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences, AXIS, EPG Health, JNCC/Harborside, and I3 Health. The remaining authors declare no conflicts of interest.
Figures
References
-
- Wolf J., Seto T., Han J.Y., et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383:944–957. - PubMed
-
- Recondo G., Bahcall M., Spurr L.F., et al. Molecular mechanisms of acquired resistance to MET tyrosine kinase inhibitors in patients with MET exon 14-mutant NSCLC. Clin Cancer Res. 2020;26:2615–2625. - PubMed
-
- Engstrom L.D., Aranda R., Lee M., et al. Glesatinib exhibits antitumor activity in lung cancer models and patients harboring MET Exon 14 mutations and overcomes mutation-mediated resistance to Type I MET inhibitors in nonclinical models. Clin Cancer Res. 2017;23:6661–6672. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

