Prediction of the efficacy and clinical prognosis of first-line EGFR-tyrosine kinase inhibitors in non-small cell lung cancer patients based on ΔCt values derived from the super-amplification refractory mutation system (ARMS): a real-world retrospective study
- PMID: 40688317
- PMCID: PMC12268672
- DOI: 10.21037/jtd-2025-97
Prediction of the efficacy and clinical prognosis of first-line EGFR-tyrosine kinase inhibitors in non-small cell lung cancer patients based on ΔCt values derived from the super-amplification refractory mutation system (ARMS): a real-world retrospective study
Abstract
Background: Lung cancer, especially non-small cell lung cancer (NSCLC), is a leading cause of cancer mortality. Epidermal growth factor receptor (EGFR) mutations drive NSCLC progression but also sensitize tumors to EGFR-tyrosine kinase inhibitors (TKIs). However, the response rate to targeted therapy is only 70%, and most patients experience disease progression 9 to 14 months after first- or second-generation EGFR-TKI treatment. This study aims to examine the association between super-amplification refractory mutation system (ARMS)-derived ΔCt values [mutant DNA cycle threshold (Ct) value relative to the endogenous reference gene (Ct) value] and EGFR mutation (EGFRm) abundance in predicting the efficacy and prognosis of EGFR-TKIs in NSCLC patients.
Methods: The present retrospective research encompassed 139 patients with stage IIIB-IV NSCLC treated with EGFR-TKIs. Patients were categorized based on super-ARMS ΔCt values and Kaplan-Meier, and Cox regression models were used to evaluate the outcomes in survival and independent influencing factors, thus establishing the optimal ΔCt value for EGFR-TKIs response.
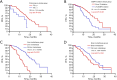
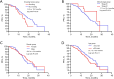
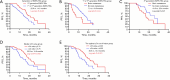
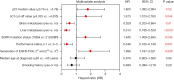
Results: High mutation abundance, defined by ΔCt ≤3.76, was correlated with increased objective response rate (ORR) (61.2% vs. 36.8%, P=0.003) and longer median progression-free survival (mPFS) (20.9 vs. 15.8 months, log-rank P=0.005) compared to low abundance. The optimal ΔCt cut-off predictive of EGFR-TKIs response was 4.335. Patients with ΔCt ≤4.335 demonstrated superior ORR (64.6% vs. 28.1%, P<0.001) and mPFS (20.9 vs. 13.5 months, log-rank P<0.001) compared to those with ΔCt >4.335. Multivariate Cox analysis identified median ΔCt value group (ΔCt ≤3.76 or ΔCt >3.76), the optimal ΔCt cut-off value group (ΔCt ≤4.335 or ΔCt >4.335), brain metastasis, liver metastasis, EGFRm status, performance status (PS) score, and the generation of EGFR-TKIs as independent predictors of PFS in first-line EGFR-TKIs-treated patients.
Conclusions: Stratification based on ΔCt values derived from the super-ARMS system can predict the efficacy and clinical prognosis of first-line EGFR-TKI treatment in NSCLC patients. Additionally, higher mutation abundance may contribute to the superior efficacy and prognosis of EGFR-TKIs in patients with exon 19 deletions compared to those with the 21L858R mutation.
Keywords: Non-small cell lung cancer (NSCLC); circulating tumor DNA (ctDNA); epidermal growth factor receptor (EGFR); super-amplification refractory mutation system (super-ARMS); tyrosine kinase inhibitors (TKIs).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-2025-97/coif). The authors have no conflicts of interest to declare.
Figures
References
-
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. 10.1093/annonc/mdv276 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
