Hyperspectral Imaging Accurately Detects Renal Malperfusion Due to High Intrarenal Pressure
- PMID: 40688377
- PMCID: PMC12274906
- DOI: 10.1016/j.euros.2025.06.007
Hyperspectral Imaging Accurately Detects Renal Malperfusion Due to High Intrarenal Pressure
Abstract
Background and objective: High intrarenal pressure (IRP) is a significant concern in both endoscopic procedures and acute hydronephrosis, and may cause renal parenchymal damage, forniceal rupture, and long-term impaired renal function. Its pathomechanism and effect on renal perfusion patterns remain undetermined. This study investigates the impact of elevated IRP on renal perfusion and oxygen saturation (StO2) using hyperspectral imaging (HSI).
Methods: In vivo experiments were conducted on porcine models establishing hydronephrosis on specific IRP levels (30, 50, 70, and 90 mmHg) by pressure-controlled infusion of crystalloid solution into the ureter after distal ureteral clamping. HSI data were recorded at baseline, during IRP application, and after release to measure hydronephrosis-induced changes in reflectance and perfusion in a total of 501 recordings. The results were compared with spectral patterns of renal malperfusion states from previous internal studies. In total, data of 73 pigs and 1744 HSI recordings were included.
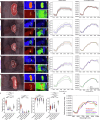
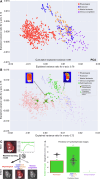
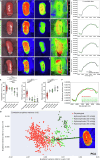
Key findings and limitations: Elevated IRP significantly affected renal perfusion and oxygenation. StO2 decreased from 70.3% ± 10.9% (physiological) to 39.9% ± 9.5% in hydronephrotic kidneys. Perfusion values in hydronephrosis decreased significantly at the renal poles (6.5% ± 4.0%) compared with physiological values (34.8% ± 7.5%). A principal component analysis and machine learning classification confirmed distinct malperfusion states, with hydronephrosis resembling ischemic conditions.
Conclusions and clinical implications: HSI revealed that high IRP reduces renal oxygenation and perfusion, with the poles being disproportionately affected. The results from this study provide quantitative evidence of perfusion restriction and ischemic conditions as the pathomechanism behind hydronephrosis-induced kidney damage. These findings underscore the importance of monitoring IRP during endourological procedures to mitigate renal damage and associated complications.
Patient summary: High pressure in the kidneys during surgery or kidney disease can severely reduce blood flow and oxygen, causing damage. This study used a special camera to show this damage, especially at the end of the kidney. These findings highlight the importance of monitoring kidney pressure carefully during procedures to prevent damage to the kidney.
Keywords: Animal study; Hydronephrosis; Hyperspectral imaging; Intrarenal pressure; Porcine model; Urology.
© 2025 The Author(s).
Figures


Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Intrarenal pressure gradient in an ex-vivo porcine kidney model.Int J Surg. 2025 Jul 1;111(7):4234-4238. doi: 10.1097/JS9.0000000000002499. Epub 2025 May 20. Int J Surg. 2025. PMID: 40391964
-
The exponential relationship between raised intrarenal pressure and bacteraemia.BJU Int. 2025 Jul 24. doi: 10.1111/bju.16856. Online ahead of print. BJU Int. 2025. PMID: 40704894
-
Normothermic and hypothermic machine perfusion preservation versus static cold storage for deceased donor kidney transplantation.Cochrane Database Syst Rev. 2024 Jul 9;7(7):CD011671. doi: 10.1002/14651858.CD011671.pub3. Cochrane Database Syst Rev. 2024. PMID: 38979743 Free PMC article.
-
Intrarenal pressure variations during flexible ureteroscopy in a porcine kidney model: impact of ureteral access sheath types and irrigation methods.World J Urol. 2025 Aug 18;43(1):501. doi: 10.1007/s00345-025-05857-1. World J Urol. 2025. PMID: 40824319
References
-
- Lee M.S., Connors B.A., Agarwal D.K., et al. Determining the threshold of acute renal parenchymal damage for intrarenal pressure during flexible ureteroscopy using an in vivo pig model. World J Urol. 2022;40:2675–2681. - PubMed
-
- Fung L.C., Atala A. Constant elevation in renal pelvic pressure induces an increase in urinary N-acetyl-beta-D-glucosaminidase in a nonobstructive porcine model. J Urol. 1998;159:212–216. - PubMed
LinkOut - more resources
Full Text Sources

