Cell retention in scalable, perfusion-based mesenchymal stem cell expansion processes: a proof of concept
- PMID: 40688478
- PMCID: PMC12271218
- DOI: 10.3389/fbioe.2025.1611703
Cell retention in scalable, perfusion-based mesenchymal stem cell expansion processes: a proof of concept
Abstract
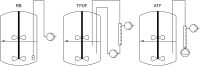
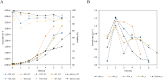
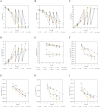
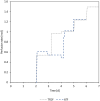
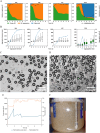
The production of clinically relevant quantities of human mesenchymal stromal cells (hMSCs) requires scalable and intensified manufacturing processes. For this reason, the applicability of alternating tangential flow filtration (ATF) and tangential flow depth filtration (TFDF) based cell retention systems for hMSC expansion on microcarriers (MCs) in perfusion mode was assessed. The processes were conducted in stirred tank bioreactors at a scale of 1.8 L and compared with repeated-batch cultivations. In the perfusion and repeated-batch control cultivations, competitive viable cell concentrations of ≈2.9 · 106 cells mL-1 were reached within a cultivation period of 5-7 days, resulting in an expansion factor of 41-57. The main difference between the operation modi was the aggregation behavior of the MCs. While the median MC aggregate diameter in the repeated-batch cultivation reached 470 μm, the ATF cell retention device constrained aggregate size to a median diameter of 250 µm. In the TFDF cultivation, the shear forces in the recirculation loop stripped most of the hMSCs from the MCs, resulting in the formation of spheroids that continued to proliferate, albeit at a decreased rate. While perfusion operation did not lead to increased productivity in this proof-of-concept study, manual handling and therefore contamination risk were reduced by replacing the repeated-batch process's daily 80% medium exchanges with automated perfusion operation. Additionally, the ATF system was shown to be useful for medium removal and washing of the MCs prior to adding the harvesting solution, which is highly valuable for cultivations conducted at larger scales. While the feasibility of ATF based cell retention for MC expansion processes could be demonstrated, increased growth area to medium ratios, i.e., higher MC concentrations, still need to be investigated to leverage the full potential of the perfusion process mode.
Keywords: alternating tangential flow filtration (ATF); hMSC; microcarrier; perfusion; single-use technology (SUT); stirred tank bioreactor; tangential flow filtration (TFF).
Copyright © 2025 Schneider, Gopalakrishnan, Teale and Eibl.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Scalable, High-Density Expansion of Human Mesenchymal Stem Cells on Microcarriers Using the Bach Impeller in Stirred-Tank Reactors.Biotechnol Bioeng. 2025 Jul 17. doi: 10.1002/bit.70025. Online ahead of print. Biotechnol Bioeng. 2025. PMID: 40678872
-
Developing an ultra-intensified fed-batch cell culture process with greatly improved performance and productivity.Biotechnol Bioeng. 2024 Feb;121(2):696-709. doi: 10.1002/bit.28605. Epub 2023 Nov 23. Biotechnol Bioeng. 2024. PMID: 37994547
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Technological aids for the rehabilitation of memory and executive functioning in children and adolescents with acquired brain injury.Cochrane Database Syst Rev. 2016 Jul 1;7(7):CD011020. doi: 10.1002/14651858.CD011020.pub2. Cochrane Database Syst Rev. 2016. PMID: 27364851 Free PMC article.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
References
-
- Barekzai J., Friedrich J., Okpara M., Refflinghaus L., Eckhardt D., Czermak P., et al. (2023). Dynamic expansion of mesenchymal stem/stromal cells in a stirred tank bioreactor promotes the release of potent extracellular vesicles. AIMS Bioeng. 10 (3), 240–264. 10.3934/bioeng.2023016 - DOI
-
- Borys B. S., Dang T., So T., Rohani L., Revay T., Walsh T., et al. (2021). Overcoming bioprocess bottlenecks in the large-scale expansion of high-quality hiPSC aggregates in vertical-wheel stirred suspension bioreactors. Stem Cell Res. and Ther. 12, 55. 10.1186/s13287-020-02109-4 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

