Open-source tubing-free impeller pump platform for controlled recirculating fluid flow for microfluidics and organs-on-chip
- PMID: 40688519
- PMCID: PMC12274319
- DOI: 10.1016/j.ohx.2025.e00673
Open-source tubing-free impeller pump platform for controlled recirculating fluid flow for microfluidics and organs-on-chip
Abstract
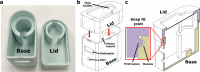
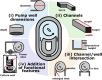
Fluid flow is utilized in many microscale technologies, including microfluidic chemical reactors, diagnostics, and organs-on-chip (OOCs). In particular, OOCs may rely on fluid flow for nutrient delivery, cellular communication, and application of shear stress. In order for microscale flow systems to be readily adopted by non-experts, a tubing-free, user-friendly pump would be useful, particularly one that is simple to use, affordable, and compatible with cell culture incubators. To address these needs, here we share the design and fabrication of an impeller pump platform that provides recirculating fluid flow through a microfluidic loop without the need for tubing connections. Flow is driven by rotating a magnetic stir bar or 3D-printed impeller in a pump well, using magnets mounted on a DC motor. The DC motors used produce negligible heat output in a compact system, making it compatible with cell culture incubators. The pump platform accommodates user-defined microfluidic or OOC device geometries, which may be easily customized by 3D printing. Furthermore, the system is easily assembled from low-cost materials and simple circuitry by someone with no prior training. We demonstrate the ability of the platform to drive recirculating fluid flow in a microfluidic device at well-characterized flow velocities ranging from µm/s to mm/s for use with microfluidic technologies. Though designed with OOCs in mind, we envision that this platform will enable users from ranging disciplines to incorporate fluid flow in customized microscale technologies.
Keywords: 3D printing; Bioreactor; Micropump; Multi-organ-on-chip; Stir bar.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Leung C.M., de Haan P., Ronaldson-Bouchard K., Kim G.-A., Ko J., Rho H.S., Chen Z., Habibovic P., Jeon N.L., Takayama S., Shuler M.L., Vunjak-Novakovic G., Frey O., Verpoorte E., Toh Y.-C. Nat. Rev; Methods Primer: 2022. A guide to the organ-on-a-chip.
-
- Ortiz-Cárdenas J.E., Zatorski J.M., Arneja A., Montalbine A.N., Munson J.M., Luckey C.J., Pompano R.R. Towards spatially-organized organs-on-chip: photopatterning cell-laden thiol-ene and methacryloyl hydrogels in a microfluidic device. Organs–-Chip 4. 2022 doi: 10.1016/j.ooc.2022.100018. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

