Development of stromal corneal grafts using a novel decellularization method with sodium cocoyl glutamate on GGTA1/ CMAH/ β4GalNT2 knock-out porcine corneas
- PMID: 40689077
- PMCID: PMC12272801
- DOI: 10.21037/atm-25-3
Development of stromal corneal grafts using a novel decellularization method with sodium cocoyl glutamate on GGTA1/ CMAH/ β4GalNT2 knock-out porcine corneas
Abstract
Background: Diseases of the human cornea often necessitate corneal transplantation. However, donor corneas are not always readily available, leaving many patients waiting for donated corneas. Porcine corneas are a promising alternative to human donor corneas due to their close anatomical and physiological similarities. In this study, we produced GGTA1/CMAH/β4GalNT2 knockout pigs [triple knockout (TKO)] to minimize immune rejection. We investigated the efficacy and safety of a novel corneal decellularization process using sodium cocoyl glutamate (SCG) and supernuclease (SN).
Methods: We harvested cornea stromal grafts from 2-month-old TKO pigs, decellularized them using SCG and SN. The optical transparency, DNA content, collagen content, glycosaminoglycan content, and tensile strength of the decellularized corneas were measured. The in vivo safety and efficacy of the decellularized corneas were evaluated by transplanting them into the stromal pockets of rabbit corneas. Comparisons between wild type (WT) and TKO corneas, both decellularized and non-decellularized, were performed over a 4-week period post-transplantation.
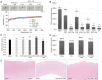
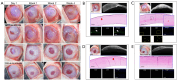
Results: Compared to a previous method using sodium N-lauroyl glutamate (SLG), the method using 0.5% SCG and SN more effectively removed DNA from the corneal stroma without significantly changing tensile strength, transparency, collagen, or glycosaminoglycan content. When decellularized corneas were implanted into corneal stromal pockets of rabbits, at 4 weeks post-surgery, decellularized corneas from WT pigs showed significant corneal neovascularization and opacity. In contrast, those from TKO pigs with 0.5% SCG plus SN decellularization maintained good transparency with minimal vascularization.
Conclusions: Corneas from TKO pigs could be successfully decellularized using 0.5% SCG plus SN method, showing promising results after transplanting them into rabbit corneas.
Keywords: Cornea; decellularization; knock out; pig; sodium cocoyl glutamate (SCG).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-25-3/coif). J.T.K. and P.H.C. are employees of MGENSolutions Co., Ltd. The other authors have no conflicts of interest to declare.
Figures





References
LinkOut - more resources
Full Text Sources
