Antifibrotic therapies for metabolic dysfunction-associated steatotic liver disease
- PMID: 40689145
- PMCID: PMC12276452
- DOI: 10.1016/j.jhepr.2025.101421
Antifibrotic therapies for metabolic dysfunction-associated steatotic liver disease
Abstract
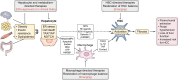
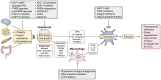
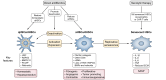
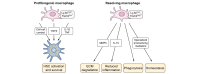
Metabolic dysfunction-associated steatotic liver disease (MASLD) affects more than a quarter of the adult population worldwide. MASLD can progress to metabolic dysfunction-associated steatohepatitis (MASH), which is associated with increased risk of progression to liver fibrosis, cirrhosis and hepatocellular carcinoma, as well as cardiovascular complications. The pathogenesis of MASLD is complex and initiated by altered metabolic signalling circuits between the adipose tissue, muscle, gut and liver. Liver fibrosis is largely driven by the crosstalk of steatotic hepatocytes with macrophages and hepatic stellate cells and constitutes the primary determinant of outcomes in MASLD. Therefore, fibrosis regression is a key therapeutic goal for MASH therapies. Here, we review therapeutic strategies that directly or indirectly reduce liver fibrosis and discuss novel therapeutic concepts. Among these, the targeting of hepatocytes and metabolism have yielded fibrosis reduction in clinical trials and led to the first FDA-approved therapy for MASH. However, these therapies reduce fibrosis only in a subset of patients and have not yet shown benefits beyond the F2-F3 fibrosis stage. Direct antifibrotics and macrophage-based therapies may be more suitable for advanced stages of MASH, but are still in the developmental stage. The arsenal of therapies for MASLD is rapidly expanding and includes macrophage transplantation, hepatocyte-specific oligonucleotides, as well as CAR T cell-based therapies. Integrating these novel therapeutic concepts into stage-specific and/or combination therapies targeting divergent pathogenic mechanisms and cell types is the focus of ongoing research, which may lead to fibrosis reduction in a higher percentage of patients with MASH.
Keywords: HSC; Kupffer cells; NAFLD; NASH; Non-alcoholic fatty liver disease; cirrhosis; hepatocellular carcinoma; inflammation; non-alcoholic steatohepatitis; outcomes; pharmacologic; portal hypertension.
© 2025 The Authors.
Conflict of interest statement
R.F.S., F.T., A.S. and S.L.F. declare no conflicts of interest. F.T. reports research funding from AstraZeneca, MSD, Gilead, Agomab (fundings to his institution); consulting fees from AstraZeneca, Gilead, GSK, Abbvie, BMS, Ipsen, Pfizer, Novartis, Novo Nordisk, Madrigal, MSD, Sanofi, Boehringer; payment or honoraria from Gilead, AbbVie, Falk, Merz, Intercept, Sanofi, Astra Zeneca, Boehringer; support for attending meetings and/or travel from Gilead; participation in Advisory Boards from Sanofi, MSD and Pfizer. S.L.F has relationships with the companies listed below; however, these activities are unrelated to the content of this article: Consulting: 89 Bio, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, ChemomAb, Foresite Laboratories, Gordian Biotechnology, Glycotest, Glympse Bio, Hepgene, In sitro, Junevity, Korro Bio, Kriya, Laekna, Lerna Therapeutics, Macomics, Mediar, Merck, Morphic Therapeutics, North Sea Therapeutics, Ochre Bio, Overtone Therapeutics, Pfizer Pharmaceuticals, Pliant, Prosciento, RAPT, Sagimet, Satellite Bio, Seal Rock, Scholar Rock, Sunbird Bio, Surrozen, Takeda, Variant Bio. Stock options: Escient, Galectin, Galmed, Genfit, Gordian Biotechnology, Hepgene, Junevity, Lifemax, Metacrine, Morphic Therapeutics, North Sea, Ochre Bio, Therapeutics, Scholar Rock, and Sunbird Bio. Research Activities with Commercial Entities: Abalone Bio (SBIR Grant) and Novo Nordisk. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Similar articles
-
Multi-modal analysis of human hepatic stellate cells identifies novel therapeutic targets for metabolic dysfunction-associated steatotic liver disease.J Hepatol. 2025 May;82(5):882-897. doi: 10.1016/j.jhep.2024.10.044. Epub 2024 Nov 8. J Hepatol. 2025. PMID: 39522884 Free PMC article.
-
Phenotypes and ontogeny of senescent hepatic stellate cells in metabolic dysfunction-associated steatohepatitis.J Hepatol. 2024 Aug;81(2):207-217. doi: 10.1016/j.jhep.2024.03.014. Epub 2024 Mar 18. J Hepatol. 2024. PMID: 38508241 Free PMC article.
-
Modeling the health and economic impact of pharmacologic therapies for MASLD in the United States.J Hepatol. 2025 Jul;83(1):21-30. doi: 10.1016/j.jhep.2025.01.009. Epub 2025 Jan 18. J Hepatol. 2025. PMID: 39832655
-
Ultrasonography for diagnosis of alcoholic cirrhosis in people with alcoholic liver disease.Cochrane Database Syst Rev. 2016 Mar 2;3(3):CD011602. doi: 10.1002/14651858.CD011602.pub2. Cochrane Database Syst Rev. 2016. PMID: 26934429 Free PMC article.
-
Pathological Evolution and Internal Medicine Management of Nonalcoholic Fatty Liver Disease (NAFLD) in the Era of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD).Cureus. 2025 Jun 29;17(6):e86963. doi: 10.7759/cureus.86963. eCollection 2025 Jun. Cureus. 2025. PMID: 40734888 Free PMC article. Review.
Cited by
-
Oxy210 Inhibits Hepatic Expression of Senescence-Associated, Pro-Fibrotic, and Pro-Inflammatory Genes in Mice During Development of MASH and in Hepatocytes In Vitro.Cells. 2025 Aug 2;14(15):1191. doi: 10.3390/cells14151191. Cells. 2025. PMID: 40801623 Free PMC article.
References
-
- Israelsen M., Francque S., Tsochatzis E.A., et al. Steatotic liver disease. Lancet. 2024;404:1761–1778. - PubMed
-
- Amini-Salehi E., Letafatkar N., Norouzi N., et al. Global prevalence of nonalcoholic fatty liver disease: an updated review meta-analysis comprising a population of 78 million from 38 countries. Arch Med Res. 2024;55 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

