Identification of novel angiotensin I-converting enzyme inhibitory peptides from walnut (Juglans sigillata Dode) protein hydrolysates and their vascular endothelial protective properties: In silico and in vitro studies
- PMID: 40689293
- PMCID: PMC12273483
- DOI: 10.1016/j.crfs.2025.101135
Identification of novel angiotensin I-converting enzyme inhibitory peptides from walnut (Juglans sigillata Dode) protein hydrolysates and their vascular endothelial protective properties: In silico and in vitro studies
Abstract
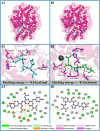
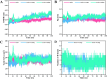
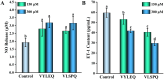
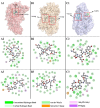
The objective of this study was to explore novel functional peptides derived from walnut protein isolate (WPI) that exhibited gastrointestinal stability, angiotensin I-converting enzyme (ACE1) inhibitory activity, and endothelial protective properties. Among the 81 detected peptides, 36 peptides with Glu, Leu, Pro, and Asp as the predominant residues were predicted to exhibit ACE1 inhibitory activity. Four promising peptides (TLEPT, TLEPN, VYLEQ, and VLSPQ) were selected for further evaluation based on predicted bioactivity and abundance. In vitro assays confirmed significant ACE1 inhibitory activity, with VYLEQ and VLSPQ demonstrating exceptional potency, exhibiting IC50 values of 27.88 ± 2.45 and 104.75 ± 7.62 μM, respectively. Molecular docking and dynamics simulations further revealed that VYLEQ displayed stronger binding affinity to ACE1 (-8.8 kcal/mol) compared to VLSPQ (-8.4 kcal/mol), facilitated by hydrogen bonding, van der Waals forces, and interactions with Zn2+ at the enzyme's active site, contributing to enhanced stability of the ACE1-VYLEQ complex. Moreover, the EA.hy926 cell assay showed that both peptides promoted nitric oxide (NO) release and inhibited endothelin-1 (ET-1) secretion. Molecular docking revealed that both peptides interact with key proteins that regulate NO and ET-1 mainly through van der Waals forces and hydrogen bonding. These findings suggest their potential benefit in antihypertensive effects. The findings of this study have the potential to increase the economic value of skim walnut meal, and to provide a theoretical foundation for the development of novel functional foods that may have a beneficial effect on the health of patients suffering from hypertension.
Keywords: ACE1 inhibitory peptides; Gastrointestinal digestion; Human endothelial cell; Molecular docking; Molecular dynamics simulation; Walnut protein isolate.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Chen M., Wang L., Zheng C., Ma A., Hu K., Xiang A., Sun Z., Xie B., Xiong G., Shi L., Chen S., Wu W. Novel ACE1 inhibitory peptides derived from bighead carp (Aristichthys nobilis) hydrolysates: screening, inhibition mechanisms and the bioconjugation effect with graphene oxide. Food Biosci. 2023;52 doi: 10.1016/j.fbio.2023.102399. - DOI
-
- Dong Y., Dai Z. Physicochemical properties, biological activities, and peptidome profile of grass carp swim bladder collagen hydrolysates. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2023;189 doi: 10.1016/j.lwt.2023.115544. - DOI
LinkOut - more resources
Full Text Sources

