The cell-adhesion molecule Echinoid promotes tissue survival and separately restricts tissue overgrowth
- PMID: 40689564
- PMCID: PMC12377819
- DOI: 10.1242/dev.204572
The cell-adhesion molecule Echinoid promotes tissue survival and separately restricts tissue overgrowth
Abstract
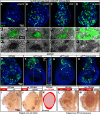
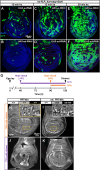
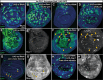
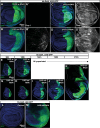
The growth and survival of cells depends both on their intrinsic properties and interactions with their neighbors. In a screen of genes encoding cell-surface proteins for knockdowns that affect clone size or shape in mosaic Drosophila imaginal discs, we found that clones with reduced echinoid (ed) function are fewer and smaller, and are frequently eliminated during development. This elimination results, in significant part, from increased levels of apoptosis due to decreased Diap1 protein. We found that Hippo pathway activity is not decreased in ed mutant cells, as previously claimed, but is decreased in some of their immediate wild-type neighbors, consistent with the observed elimination of ed clones by a mechanism resembling cell competition. In contrast to the underrepresentation of ed clones, discs or compartments composed of mostly ed mutant tissue overgrow, despite having increased levels of apoptosis. The overgrowth results from a failure to arrest growth at the appropriate final size during an extended larval stage. Thus, ed has two distinct functions: an anti-apoptotic function via maintenance of Diap1 levels, and a function to arrest growth at the appropriate final size.
Keywords: Adhesion; Cell competition; Echinoid; Growth; Hippo.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



Update of
-
The cell adhesion molecule Echinoid promotes tissue survival and separately restricts tissue overgrowth in Drosophila imaginal discs.bioRxiv [Preprint]. 2023 Aug 6:2023.08.04.552072. doi: 10.1101/2023.08.04.552072. bioRxiv. 2023. Update in: Development. 2025 Aug 1;152(15):dev204572. doi: 10.1242/dev.204572. PMID: 37577631 Free PMC article. Updated. Preprint.
References
-
- Adachi-Yamada, T., Harumoto, T., Sakurai, K., Ueda, R., Saigo, K., O'Connor, M. B. and Nakato, H. (2005). Wing-to-leg homeosis by spineless causes apoptosis regulated by FISH-lips, a novel leucine-rich repeat transmembrane protein. Mol. Cell. Biol. 25, 3140-3150. 10.1128/MCB.25.8.3140-3150.2005 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

