Correlation of Multimodal Clinical Imaging With the Whole-Slide- and Superresolution-Based Immunohistological Structure of the Corneal Limbal Stroma
- PMID: 40689725
- PMCID: PMC12302048
- DOI: 10.1167/iovs.66.9.54
Correlation of Multimodal Clinical Imaging With the Whole-Slide- and Superresolution-Based Immunohistological Structure of the Corneal Limbal Stroma
Abstract
Purpose: To identify structural landmarks in the limbal stroma by anterior segment optical coherence tomography (asOCT), asOCT angiography (asOCTa) and in vivo confocal microscopy (IVCM), and correlate findings with immunofluorescence microscopy (IFM).
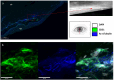
Methods: The corneal limbus of healthy individuals was examined by IVCM, asOCT, and asOCTa. IFM was performed in cadaveric cornea samples sectioned in clinical imaging planes, with putative markers for limbal niche cells (CD90, N-cadherin, SSEA-4), blood/lymphatic vessels (CD31), nerves (acetyl-α-tubulin, β-III tubulin), limbal epithelial progenitor cells (p63), and hyaluronic acid (HA).
Results: On asOCT, asOCTa, and IVCM superficial and deep limbal vessels could be identified that terminated in the marginal corneal arcade (MCA). Limbal stroma appeared as hyperreflective area between superficial and deep limbal blood vessels. The same blood vessel patterns were identified by IFM. HA localization was identical to hyperreflective stromal structures. Nerves and putative niche cells localized around limbal vessels and were in close contact with the basal limbal epithelium at the level of the MCA. Limbal epithelium was hyperreflective and thinned in the elderly (>60 years, 122.6 ± 36.6 µm; <60 years, 139.9 ± 34.4 µm; P = 0.025) resulting in less visible palisades of Vogt on asOCT (>60 years, 82.6% visible; <60 years, 100% visible; P = 0.011).
Conclusions: Our study proves that limbal vessel plexuses can serve as landmarks to identify corresponding structures in various clinical imaging modalities. The proximity of blood vessels, niche cells, and nerves, confirmed by IFM, may suggest that limbal vascular damage occurs together with niche cell and neural loss. The IFM correlations provided by this study help to detect healthy limbal structures and aid the diagnosis of diseased corneas.
Conflict of interest statement
Disclosure:
Figures






References
-
- Schlotzer-Schrehardt U, Kruse FE.. Identification and characterization of limbal stem cells. Exp Eye Res. 2005; 81(3): 247–264. - PubMed
-
- Bonnet C, Gonzalez S, Deng SX.. Limbal stem cell therapy. Curr Opin Ophthalmol. 2024; 35(4): 309–314. - PubMed
-
- Kenyon KR, Tseng SC.. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989; 96(5): 709–722; discussion 722–703. - PubMed
-
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M.. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997; 349(9057): 990–993. - PubMed
-
- Higa K, Higuchi J, Kimoto R, et al.. Human corneal limbal organoids maintaining limbal stem cell niche function. Stem Cell Res. 2020; 49: 102012. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

