Reversion of a RND transporter pseudogene reveals latent stress resistance potential in Brucella ovis
- PMID: 40690529
- PMCID: PMC12306736
- DOI: 10.1371/journal.pgen.1011795
Reversion of a RND transporter pseudogene reveals latent stress resistance potential in Brucella ovis
Abstract
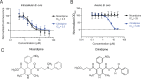
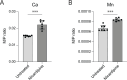
Small-molecule screens can advance therapeutic discovery and uncover new features of pathogen biology. Through a luminescence-based screen, we identified clinically approved dihydropyridines that impaired fitness of the intracellular pathogen Brucella ovis in mammalian phagocytes. Given that dihydropyridines block mammalian L-type calcium channels, and based on our observation that drug treatment perturbed calcium and manganese levels in host phagocytes, we initially hypothesized a host-directed mechanism of action. However, dose-response assays in axenic medium showed that dihydropyridines have direct antimicrobial effects. To explore the genetic basis of dihydropyridine sensitivity, we selected for B. ovis mutants capable of growing in the presence of cilnidipine, a representative compound from this drug class. Cilnidipine-resistant mutants harbored single-nucleotide deletions in the bepE transporter pseudogene that restored its open reading frame, enabling expression of a functional RND-family transporter. B. ovis is a host-restricted ovine pathogen that has experienced significant pseudogenization in its recent evolutionary history. Reversion mutations that restored the open reading frame of the bepE pseudogene increased B. ovis resistance not only to dihydropyridines but also to a broad range of cell envelope-disrupting agents. Conversely, deleting bepE in Brucella abortus, a closely related zoonotic species that retains an intact version of the gene, increased its sensitivity to envelope disruptors in vitro and to cilnidipine in the intracellular niche. We conclude that bepE is a key determinant of chemical stress resistance in Brucella spp., and that its pseudogenization in B. ovis contributes to the documented hypersensitivity of this host-restricted lineage to chemical stressors.
Copyright: © 2025 Kim et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: S.C. is a prokaryotic genetics section editor for this journal. This competing interest will not alter adherence to PLOS policies on sharing data and materials.
Figures

Update of
-
Reversion of a RND transporter pseudogene uncovers latent stress resistance in Brucella ovis.bioRxiv [Preprint]. 2025 May 10:2025.05.10.653276. doi: 10.1101/2025.05.10.653276. bioRxiv. 2025. Update in: PLoS Genet. 2025 Jul 21;21(7):e1011795. doi: 10.1371/journal.pgen.1011795. PMID: 40655013 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

