RACK1A positively regulates opening of the apical hook in Arabidopsis thaliana via suppression of its auxin response gradient
- PMID: 40690664
- PMCID: PMC12318229
- DOI: 10.1073/pnas.2407224122
RACK1A positively regulates opening of the apical hook in Arabidopsis thaliana via suppression of its auxin response gradient
Abstract
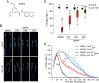
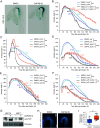
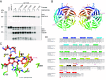
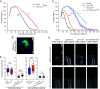
Apical hook development is an ideal model for studying differential growth in plants and is controlled by complex phytohormonal crosstalk, with auxin being the major player. Here, we identified a bioactive small molecule that decelerates apical hook opening in Arabidopsis thaliana. Our genetic studies suggest that this molecule enhances or maintains the auxin maximum found in the inner hook side and requires certain auxin signaling components to modulate apical hook opening. Using biochemical approaches, we then revealed the WD40 repeat scaffold protein RECEPTOR FOR ACTIVATED C KINASE 1A (RACK1A) as a direct target of this compound. We present data in support of RACK1A playing a positive role in apical hook opening by activating specific auxin signaling mechanisms and negatively regulating the differential auxin response gradient across the hook, thereby adjusting differential cell growth, an essential process for organ structure and function in plants.
Keywords: Arabidopsis; apical hook; auxin; differential cell growth.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
-
- Raz V., Ecker J. R., Regulation of differential growth in the apical hook of Arabidopsis. Development 126, 3661–3668 (1999). - PubMed
-
- Silk W. K., Erickson R. O., Kinematics of hypocotyl curvature. Am. J. Bot. 65, 310–319 (1978).
MeSH terms
Substances
Grants and funding
- R35 GM136338/GM/NIGMS NIH HHS/United States
- R35GM136338/Foundation for the National Institutes of Health (FNIH)
- CTS: 13: 378/Tryggers Foundation
- FATE 2022.0029/Knut och Alice Wallenbergs Stiftelse (Knut and Alice Wallenberg Foundation)
- ERC-2024-SyG STARMORPH 101166880/EC | European Research Council (ERC)
- VR 2016-00768/Vetenskapsrådet (VR)
- KAW 2016.0352/Knut och Alice Wallenbergs Stiftelse (Knut and Alice Wallenberg Foundation)
- IGA_PrF_2023_031/International Grant Agency of Palacky University
- VR 2020-03420/Vetenskapsrådet (VR)
- JCK-1732/Kempestiftelserna (Kempe Foundations)
- KAW 2020.0240/Knut och Alice Wallenbergs Stiftelse (Knut and Alice Wallenberg Foundation)
LinkOut - more resources
Full Text Sources

