Immunoglobulin prophylaxis should be initiated after bispecific antibody therapy in multiple myeloma, regardless of IgG levels
- PMID: 40690761
- PMCID: PMC12466225
- DOI: 10.1182/bloodadvances.2025016490
Immunoglobulin prophylaxis should be initiated after bispecific antibody therapy in multiple myeloma, regardless of IgG levels
Abstract
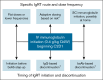
Bispecific antibodies (bsAbs), such as teclistamab, elranatamab, linvoseltamab, and talquetamab, have impressive efficacy in multiple myeloma (MM) but come with substantial infectious risks that do not dissipate over time. Immunoglobulin replacement therapy (IgRT), which includes IV and subcutaneous (SC) immunoglobulins, may lower these risks. In this viewpoint, we contrast primary IgRT prophylaxis (initiation regardless of IgG levels) with preemptive IgRT treatment (initiation only once IgG levels fall below a certain threshold) in this setting. We make evidence-based arguments for primary prophylaxis as a safer and simpler approach than preemptive IgG-guided IgRT. We also discuss strategies to improve the cost-effectiveness of IV and SC immunoglobulins across the world. Given the overwhelmingly favorable benefit-risk profile of IgRT, coupled with the limitations inherent to IgG measurements in MM, withholding IgRT access based on arbitrary IgG thresholds is neither scientifically sound nor clinically appropriate for patients with MM who are receiving bsAb therapy.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.B. reports serving as a consultant for AbbVie, Adaptive Biotech, Bristol Myers Squibb (BMS), Caribou Biosciences, Genentech, Gilead/Kite, GlaxoSmithKline, Janssen, Karyopharm, Legend Biotech, Pfizer, Poseida Therapeutics, Sanofi, and SparkCures; and research funding from AbbVie, BMS, Janssen, Novartis, Pack Health, Prothena, and Sanofi. M.M. reports institutional research funding from Sanofi S.A., BMS, and Celgene Corporation; serving as a consultant for Sanofi S.A., BMS/Celgene Corporation, Pfizer, Janssen Scientific Affairs LLC, and Legend Biotech. K.R. reports research funding from Kite/Gilead; serving as a consultant for Kite/Gilead, BMS/Celgene, and CSL Behring; honoraria from Kite/Gilead, Novartis, BMS/Celgene; and travel support from Pierre Fabre and Kite/Gilead. G.K. reports serving as a consultant for BMS, Prothena, Sanofi, Kite Pharma, Janssen, and Arcellx. G.J.M. reports honoraria from Pfizer and Janssen; and research funding from Antengene. B.B. reports research funding from NATCO Pharma and Intas Pharmaceuticals. N.S.R. reports serving as a consultant for BMS, Pfizer, Janssen, Amgen, Genentech, and GlaxoSmithKline; and research funding from bluebird bio and Pfizer. The remaining authors declare no competing financial interests.
Figures
References
-
- Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387(24):2232–2244. - PubMed
-
- Vij R, Kumar SK, D'Souza A, et al. Updated safety and efficacy results of Abbv-383, a BCMA x CD3 bispecific T-cell redirecting antibody, in a first-in-human phase 1 study in patients with relapsed/refractory multiple myeloma [abstract] Blood. 2023;142(suppl 1):3378.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

