A human brain network linked to restoration of consciousness after deep brain stimulation
- PMID: 40691170
- PMCID: PMC12279989
- DOI: 10.1038/s41467-025-61988-4
A human brain network linked to restoration of consciousness after deep brain stimulation
Erratum in
-
Publisher Correction: A human brain network linked to restoration of consciousness after deep brain stimulation.Nat Commun. 2025 Aug 14;16(1):7563. doi: 10.1038/s41467-025-63046-5. Nat Commun. 2025. PMID: 40813586 Free PMC article. No abstract available.
Abstract
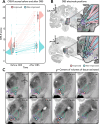
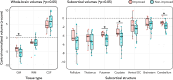
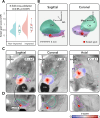
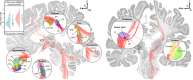
Disorders of consciousness are characterized by severe impairments in arousal and awareness. Deep brain stimulation is a potential treatment, but outcomes vary-possibly due to differences in patient characteristics, electrode placement, or the specific brain network engaged. We describe 40 patients with disorders of consciousness undergoing deep brain stimulation targeting the thalamic centromedian-parafascicular complex. Improvements in consciousness are associated with better-preserved gray matter, particularly in the striatum. Electric field modeling reveals that stimulation is most effective when it extends below the centromedian nucleus, engaging the inferior parafascicular nucleus and the adjacent ventral tegmental tract-a pathway that connects the brainstem and hypothalamus and runs along the midbrain-thalamus border. External validation analyzed show that effective stimulation engages a brain network overlapping with disrupted patterns of brain activity observed in two independent cohorts with impaired consciousness: one with arousal-impairing stroke lesions and the other with awareness-impairing seizures. Together, these findings advance the field by informing patient selection, refining stimulation targets, and identifying a brain network linked to recovery that may have broader therapeutic relevance across consciousness-impairing conditions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.E.L.W., M.R., H.F., F.L.W.V.J.S., J.T., S.B.S., J.L., M.M.J.C., K.B., M.U.F., R.J., J.E.I., P.W.C., D.F., A.D.B., B.L.E., and D.C. have no competing interests to report. M.D.F. has intellectual property on the use of brain connectivity imaging to analyze lesions and guide brain stimulation, has consulted for Magnus Medical, Soterix, Abbott, Boston Scientific, and Tal Medical, and has received research funding from Neuronetics. A.H. reports lecture fees for Boston Scientific, is a consultant for Modulight.bio, was a consultant for FxNeuromodulation and Abbott in recent years, and serves as a co-inventor on a patent granted to Charité University Medicine Berlin that covers multi-symptom D.B.S. fiber-filtering and an automated DBS parameter suggestion algorithm unrelated to this work (patent #LU103178). J.D.R. has received past consulting payments from Medtronic, Corlieve, ClearPoint, Medtronic, and NeuroPace, and currently consults for Turing Medical.
Figures
Update of
-
A human brain network linked to restoration of consciousness after deep brain stimulation.medRxiv [Preprint]. 2024 Oct 18:2024.10.17.24314458. doi: 10.1101/2024.10.17.24314458. medRxiv. 2024. Update in: Nat Commun. 2025 Jul 21;16(1):6721. doi: 10.1038/s41467-025-61988-4. PMID: 39484242 Free PMC article. Updated. Preprint.
References
-
- Giacino, J. T. et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology91, 450–460 (2018). - PMC - PubMed
-
- Kondziella, D. et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol.27, 741–756 (2020). - PubMed
-
- Fischer, D. & Edlow, B. L. Coma prognostication after acute brain injury: a review. JAMA Neurol.81, 4 (2024). - PubMed
-
- Hassler, R. et al. EEG and clinical arousal induced by bilateral long-term stimulation of pallidal systems in traumatic vigil coma. Electroencephalogr. Clin. Neurophysiol.27, 689–690 (1969). - PubMed
MeSH terms
Grants and funding
- DP2 HD101400/HD/NICHD NIH HHS/United States
- R01 NS127892/NS/NINDS NIH HHS/United States
- RF1 AG080371/AG/NIA NIH HHS/United States
- R01 AG070988/AG/NIA NIH HHS/United States
- R01 MH113929/MH/NIMH NIH HHS/United States
- RF1 MH123195/MH/NIMH NIH HHS/United States
- R21 NS123813/NS/NINDS NIH HHS/United States
- R01 MH130666/MH/NIMH NIH HHS/United States
- R21 MH126271/MH/NIMH NIH HHS/United States
- UH3 NS109557/NS/NINDS NIH HHS/United States
- R01 NS138257/NS/NINDS NIH HHS/United States
- UM1 NS132358/NS/NINDS NIH HHS/United States
- UM1 MH130981/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources

