Thermally stable metal-organic framework based iron 2,6-naphthalenedicarboxylic catalyst (Fe-NDC) for syngas conversion to olefin
- PMID: 40691208
- PMCID: PMC12280178
- DOI: 10.1038/s41598-025-09332-0
Thermally stable metal-organic framework based iron 2,6-naphthalenedicarboxylic catalyst (Fe-NDC) for syngas conversion to olefin
Abstract
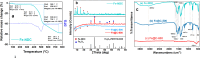
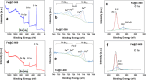
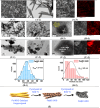
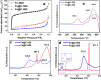
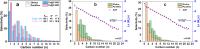
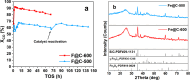
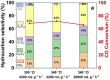
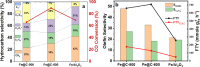
Olefins are the backbone of the petrochemical conversion industries, like polymers, plastic, lubricating oil, surfactants, and synthetic fuels. It is a wide but challenging process to customize. Metal-organic frameworks (MOFs) are highly regarded for their potential in Fischer-Tropsch synthesis (FTS), yet they often have inadequate thermal stability. This study demonstrated the remarkable potential of the Fe-NDC MOF. It maintains its initial structure until it reaches a temperature of 500 °C (Fe@C-500), which is efficient for syngas conversion to olefin. The Fe@C-500 catalyst exceeded a twofold increase in the ratio of olefin to paraffin compared to Fe@C-600 (2 vs. 0.8). The maintained structure of Fe@C-500 enhances the transport of reactants and restricts the hydrogenation of olefins. The Fe@C-500 catalyst showed ~ 50% and 27% selectivity to total olefin and light olefin, respectively, with a Fe-time yield (FTY) for light olefins of 180 mmolCO g-1Fe h-1. In contrast, Fe@C-600 exhibits a shift in product selectivity towards paraffin (~ 70%) at a lower FTY for light olefins of 130 mmolCO g-1Fe h-1. The performance of the Fe@C-500 catalyst is particularly intriguing and warrants further investigation. Retaining the porous structure of MOF-derived catalysts might greatly enhance olefin production.
Keywords: Iron catalyst; Olefin production; Syngas conversion; Thermally stable MOF.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Eldesouki, M. H., Rashed, A. E. & El-Moneim, A. A. A comprehensive overview of carbon dioxide, including emission sources, capture technologies, and the conversion into value-added products. Clean Technol. Environ. Policy25, 3131–3148 (2023).
-
- Choi, Y. H. et al. Carbon dioxide Fischer–Tropsch synthesis: A new path to carbon-neutral fuels. Appl. Catal. B202, 605–610 (2017).
-
- Braide, D., Panaritis, C., Patience, G. & Boffito, D. C. Gas to liquids (GTL) microrefinery technologies: A review and perspective on socio-economic implications. Fuel375, 125169 (2024).
-
- Dieterich, V., Buttler, A., Hanel, A., Spliethoff, H. & Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: a review. Energy Environ. Sci.13, 3207–3252 (2020).
LinkOut - more resources
Full Text Sources

