Sex dependent effects of GPR109A gene deletion in myeloid cells on bone development in mice
- PMID: 40691257
- PMCID: PMC12280110
- DOI: 10.1038/s41598-025-12017-3
Sex dependent effects of GPR109A gene deletion in myeloid cells on bone development in mice
Abstract
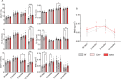
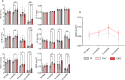
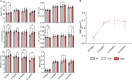
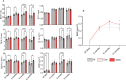
Blueberry metabolite-derived phenolic acids are thought to suppress bone resorption via interactions with the G protein-coupled receptor 109A (GPR109A). Previously, global GPR109A knockout (GPR109A-/-) mice exhibited increased bone mass and a diminished bone-protective response to phenolic acids. While GPR109A is highly expressed in osteoclast precursor macrophages, its role in bone development remains unclear. To address this, we generated a myeloid cell-specific GPR109A knockout (GPR109Aflox/flox/LysM-Cre⁺; CKO) mouse model and assessed bone phenotypes in male and female mice at 35 days, 3 months, 6 months, and 12 months using µCT. At 35 days, CKO males showed significantly increased trabecular bone in both tibia and vertebrae when compared to control genotypes (f/f, Cre⁺). However, at later time points (6 and 12 months), Cre⁺ males exhibited similar trabecular bone phenotypes compared to CKO mice. In contrast, female CKO mice displayed significantly increased trabecular bone at 6 and 12 months. Using three-point bending analysis it was found that only 12-month-old Cre⁺ and CKO male mice exhibited altered bone mechanical properties when compared to f/f mice, while for females no significant changes in bone mechanical properties were observed. These findings suggest that GPR109A regulates bone turnover pathways in a sex-specific manner.
Keywords: Bone; Osteoclast; Sexual dimorphism; µCT.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Update of
-
Sex-dependent effect of GPR109A gene deletion in myeloid cells on bone development in mice.Res Sq [Preprint]. 2025 Apr 2:rs.3.rs-6206075. doi: 10.21203/rs.3.rs-6206075/v1. Res Sq. 2025. Update in: Sci Rep. 2025 Jul 22;15(1):26515. doi: 10.1038/s41598-025-12017-3. PMID: 40235504 Free PMC article. Updated. Preprint.
Similar articles
-
Sex-dependent effect of GPR109A gene deletion in myeloid cells on bone development in mice.Res Sq [Preprint]. 2025 Apr 2:rs.3.rs-6206075. doi: 10.21203/rs.3.rs-6206075/v1. Res Sq. 2025. Update in: Sci Rep. 2025 Jul 22;15(1):26515. doi: 10.1038/s41598-025-12017-3. PMID: 40235504 Free PMC article. Updated. Preprint.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
High Fructose and High Fat Exert Different Effects on Changes in Trabecular Bone Micro-structure.J Nutr Health Aging. 2018;22(3):361-370. doi: 10.1007/s12603-017-0933-0. J Nutr Health Aging. 2018. PMID: 29484349 Free PMC article.
-
Inactivation of Invs/Nphp2 in renal epithelial cells drives infantile nephronophthisis like phenotypes in mouse.Elife. 2023 Mar 15;12:e82395. doi: 10.7554/eLife.82395. Elife. 2023. PMID: 36920028 Free PMC article.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
References
-
- Tunaru, S. et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med.9, 352–355. 10.1038/nm824 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

