Non-invasive scoring systems of liver fibrosis predict prognosis in the cohort with myocardial infarction
- PMID: 40691268
- PMCID: PMC12280198
- DOI: 10.1038/s41598-025-12583-6
Non-invasive scoring systems of liver fibrosis predict prognosis in the cohort with myocardial infarction
Abstract
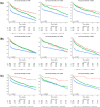
Patients with liver fibrosis and type 2 diabetes mellitus (T2DM) have an increased risk of cardiovascular events. However, long-term prognosis of liver fibrosis and T2DM after acute myocardial infarction (AMI) remain to be investigated. This study compared clinical characteristics and prognosis of AMI patients with T2DM and evidence of liver fibrosis. Patients were stratified into low, intermediate and high-risk for fibrosis, using serum-based non-invasive tests (NITs): Fibrosis-4 Index (FIB-4), Aspartate Aminotransferase to Platelet Ratio Index (APRI) and Non-alcoholic Fatty Liver Disease Fibrosis Score (NFS). The primary outcome was all-cause mortality, Kaplan-Meier curves were constructed for 5-year all-cause mortality. Cox regression analysis was used to determine the independent predictors of mortality, adjusting for confounders. Out of 3287 AMI patients, 1547 were stratified as high-risk by any NIT (mean follow-up duration 2.7 ± 2.3 years). A dose-response relationship was found with increasing mortality risk for higher APRI and NFS scores. High-risk FIB-4 also predicted mortality significantly (adjusted HR [aHR] 1.791, 95% CI 1.436-2.235, p < 0.001). High-risk FIB-4 and APRI independently predicted mortality regardless of T2DM status, while NFS only predicted mortality in T2DM patients. Following AMI, individuals stratified by FIB-4, APRI, NFS as high-risk for liver fibrosis were associated with excess long-term mortality (aHR 1.780, 95% CI 1.442-2.196, p < 0.001). Hence, readily available NITs may be beneficial in risk prognostication of AMI patients.
Keywords: Acute coronary syndrome; Aspartate aminotransferase to platelet ratio index; Fibrosis-4 index; Metabolic dysfunction-associated steatotic liver disease; Non-alcoholic fatty liver disease fibrosis score; Non-invasive tests.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The study was approved by the local institutional review committee in accordance with the revised Declaration of Helsinki (NHG Research—DSRB: 2021/00089-AMD0001). The institutional review board waived the need for written patient consent as this study involved a retrospective analysis of clinically acquired data.
Figures
References
MeSH terms
Substances
Grants and funding
- speaker's fees and research grants/Astra Zeneca, Abbott Technologies, and Boston Scientific
- NUHSRO/2022/058/RO5+6/Seed-Mar/03/NUHS Seed Fund
- MH 095:003/008-303/National Medical Research Council Research Training Fellowship
- NCSP2.0/2024/NUHS/NCWS/National University of Singapore Yong Loo Lin School of Medicine's Academic Fellowship Scheme, and the NUHS Clinician Scientist Program
LinkOut - more resources
Full Text Sources
Medical

