LONP1 regulation of mitochondrial protein folding provides insight into beta cell failure in type 2 diabetes
- PMID: 40691304
- PMCID: PMC12373512
- DOI: 10.1038/s42255-025-01333-7
LONP1 regulation of mitochondrial protein folding provides insight into beta cell failure in type 2 diabetes
Abstract
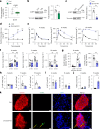
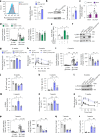
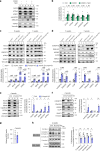
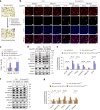
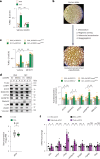
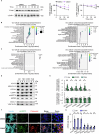
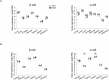
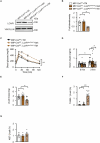
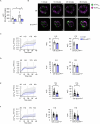
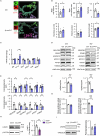
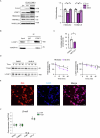
Protein misfolding is a contributor to the development of type 2 diabetes (T2D), but the specific role of impaired proteostasis is unclear. Here we show a robust accumulation of misfolded proteins in the mitochondria of human pancreatic islets from patients with T2D and elucidate its impact on β cell viability through the mitochondrial matrix protease LONP1. Quantitative proteomics studies of protein aggregates reveal that islets from donors with T2D have a signature resembling mitochondrial rather than endoplasmic reticulum protein misfolding. Loss of LONP1, a vital component of the mitochondrial proteostatic machinery, with reduced expression in the β cells of donors with T2D, yields mitochondrial protein misfolding and reduced respiratory function, leading to β cell apoptosis and hyperglycaemia. LONP1 gain of function ameliorates mitochondrial protein misfolding and restores human β cell survival after glucolipotoxicity via a protease-independent effect requiring LONP1-mitochondrial HSP70 chaperone activity. Thus, LONP1 promotes β cell survival and prevents hyperglycaemia by facilitating mitochondrial protein folding. These observations provide insights into the nature of proteotoxicity that promotes β cell loss during the pathogenesis of T2D, which could be considered as future therapeutic targets.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.A.S. has received grant funding from Ono Pharmaceutical and is a consultant for Novo Nordisk. G.A.R. has received grant funding from Sun Pharmaceuticals and Les Laboratoires Servier and is a consultant for Sun Pharmaceuticals. A.I.N. receives royalties from the University of Michigan for the sale of MSFragger and IonQuant software licences to commercial entities. All licence transactions are managed by the University of Michigan Innovation Partnerships office; all proceeds are subject to university technology transfer policy. The other authors declare no competing interests.
Figures






Update of
-
LONP1 regulation of mitochondrial protein folding provides insight into beta cell failure in type 2 diabetes.bioRxiv [Preprint]. 2024 Jun 3:2024.06.03.597215. doi: 10.1101/2024.06.03.597215. bioRxiv. 2024. Update in: Nat Metab. 2025 Aug;7(8):1570-1592. doi: 10.1038/s42255-025-01333-7. PMID: 38895283 Free PMC article. Updated. Preprint.
References
-
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell186, 243–278 (2023). - PubMed
-
- Kaushik, S. & Cuervo, A. M. Proteostasis and aging. Nat. Med.21, 1406–1415 (2015). - PubMed
-
- Marzban, L., Park, K. & Verchere, C. B. Islet amyloid polypeptide and type 2 diabetes. Exp. Gerontol.38, 347–351 (2003). - PubMed
MeSH terms
Substances
Grants and funding
- CIHR-IRSC TDP-186358/Gouvernement du Canada | Instituts de Recherche en Santé du Canada | CIHR Skin Research Training Centre (Skin Research Training Centre)
- WT212625/Z/18/Z/Wellcome Trust (Wellcome)
- MR/R022259/1/RCUK | Medical Research Council (MRC)
- R01 DK135032/DK/NIDDK NIH HHS/United States
- U24 DK098085/DK/NIDDK NIH HHS/United States
- 1-81-ACE-015/American Diabetes Association (ADA)
- R01 DK108921/DK/NIDDK NIH HHS/United States
- R01 DK118011/DK/NIDDK NIH HHS/United States
- R01 DK127270/DK/NIDDK NIH HHS/United States
- R01DK135268/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R01 DK108921, R01 DK135032, R01 DK135268, R01 DK136671, R01 DK127270, U01 DK127747, P30 DK020572/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- 1-25-PDF-126/American Diabetes Association (ADA)
- R01 DK136671, R01 DK118011/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- K01 DK133533/DK/NIDDK NIH HHS/United States
- R01 DK135268/DK/NIDDK NIH HHS/United States
- U01 DK127747/DK/NIDDK NIH HHS/United States
- I01 BX004444/BX/BLRD VA/United States
- R01 DK136671/DK/NIDDK NIH HHS/United States
- SRA-2023-1392, SRA-2024-1586/JDRF/United States
- P500PM_225305 / 1/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- BDA 16/0005485/DUK_/Diabetes UK/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- CFI 42649/Innovation, Science and Economic Development Canada
- P30 DK020572/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

