Modulation of metabolic, inflammatory and fibrotic pathways by semaglutide in metabolic dysfunction-associated steatohepatitis
- PMID: 40691365
- PMCID: PMC12443624
- DOI: 10.1038/s41591-025-03799-0
Modulation of metabolic, inflammatory and fibrotic pathways by semaglutide in metabolic dysfunction-associated steatohepatitis
Abstract
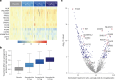
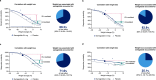
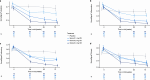
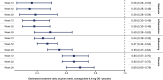
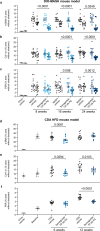
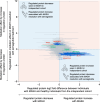
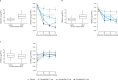
Metabolic dysfunction-associated steatohepatitis (MASH) is a chronic liver disease strongly associated with cardiometabolic risk factors. Semaglutide, a glucagon-like peptide-1 receptor agonist, improves liver histology in MASH, but the underlying signals and pathways driving semaglutide-induced MASH resolution are not well understood. Here we show that, in two preclinical MASH models, semaglutide improved histological markers of fibrosis and inflammation and reduced hepatic expression of fibrosis-related and inflammation-related gene pathways. Aptamer-based proteomic analyses of serum samples from patients with MASH in a clinical trial identified 72 proteins significantly associated with MASH resolution and semaglutide treatment, with most related to metabolism and several implicated in fibrosis and inflammation. An independent real-world cohort verified the pathophysiological relevance of this signature, showing that the same 72 proteins are differentially expressed in patients with MASH relative to healthy individuals. Taken together, these data suggest that semaglutide may revert the circulating proteome associated with MASH to the proteomic pattern observed in healthy individuals.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: E.B. has served as a consultant or advisory board member for Boehringer Ingelheim, Gilead, Intercept, Merck, Novo Nordisk, Pfizer and ProSciento and as a speaker for Gilead, Intercept, Merck, Novo Nordisk and Pfizer. E.B. also received a research grant from Gilead for fatty liver research. K.C. has received research support for the University of Florida as principal investigator from Boehringer Ingelheim, Echosens, Inventiva, LabCorp and Perspectum and has served as a consultant for Aligos Therapeutics, Arrowhead, AstraZeneca, 89bio, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Prosciento, Sagimet Biosciences, Siemens USA and Terns Pharmaceuticals. P.N.N. reports grants from Novo Nordisk and has received consulting fees from Boehringer Ingelheim, Madrigal and Novo Nordisk. P.N.N. also reports honoraria as a speaker from AiCME, Echosens and Novo Nordisk; support for attending meetings from Novo Nordisk; and participation on advisory boards for Boehringer Ingelheim, GlaxoSmithKline, Madrigal, Novo Nordisk and Sagimet. Q.M.A. is supported by the NIHR Newcastle Biomedical Research Centre and the Innovative Medicines Initiative (IMI2) program of the European Union. Q.M.A. has received consulting fees on behalf of Newcastle University from 89bio, Akero, Alimentiv, AstraZeneca, Axcella, Boehringer Ingelheim, Bristol Myers Squibb, Galmed, Genentech, Genfit, Gilead, GlaxoSmithKline, Hanmi, HistoIndex, Intercept, Inventiva, Ionis, IQVIA, Janssen, Madrigal, Medpace, Merck, NGMBio, Novartis, Novo Nordisk, PathAI, Pfizer, Pharmanest, Poxel, Prosciento, Resolution Therapeutics, Ridgeline Therapeutics, Roche, RTI, Shionogi and Terns Pharmaceuticals. He has received payment or honoraria from Fishawack, Integritas Communications, Kenes, Madrigal, Medscape, Novo Nordisk and Springer Healthcare. R.L. has served as a consultant to 89bio, Aardvark Therapeutics, Altimmune, Arrowhead Pharmaceuticals, AstraZeneca, Cascade Pharmaceuticals, Eli Lilly, Gilead, Glympse Bio, Inipharma, Intercept, Inventiva, Ionis, Janssen, Lipidio, Madrigal, Neurobo, Novo Nordisk, Merck, Pfizer, Sagimet, Takeda, Terns Pharmaceuticals and Viking Therapeutics. In addition, his institution received research grants from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galectin Therapeutics, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, Novo Nordisk, Pfizer, Sonic Incytes and Terns Pharmaceuticals. He is co-founder of LipoNexus, Inc. V.R. has received consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Madrigal, Novo Nordisk, ProSciento and Sagimet and research grants (to institution) from Merck Sharp & Dohme. V.W.-S.W. has served as a consultant or advisory board member for AbbVie, Boehringer Ingelheim, Echosens, Gilead Sciences, Intercept, Inventiva, Novo Nordisk, Pfizer, Sagimet Biosciences and TARGET PharmaSolutions and as a speaker for Abbott, AbbVie, Gilead Sciences, Novo Nordisk and Unilab. He received a research grant from Gilead Sciences and is a co-founder of Illuminatio Medical Technology Limited. He has received travel support from AbbVie and Gilead Sciences. M.J., J.N., M.S.K., K.A., K.M.B., E.D.G., M.G., L.L.G., L.M.H., G.M., L.M.N., M.S.P., A.-S.S. and L.B.K. are employees and/or stockholders of Novo Nordisk A/S.
Figures






References
-
- European public assessment report for Ozempic. European Medicines Agencyhttps://www.ema.europa.eu/en/medicines/human/EPAR/ozempic (2017).
-
- Product information - Wegovy. European Medicines Agencyhttps://www.ema.europa.eu/en/medicines/human/EPAR/wegovy (2023).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

