Gemtuzumab ozogamicin in first-line treatment of CBF-AML: insights from a retrospective multi-center analysis
- PMID: 40691504
- PMCID: PMC12380593
- DOI: 10.1038/s41375-025-02700-9
Gemtuzumab ozogamicin in first-line treatment of CBF-AML: insights from a retrospective multi-center analysis
Abstract
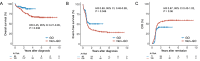
The addition of gemtuzumab ozogamicin (GO) to intensive chemotherapy (IC) has become a mainstay in treating patients with core binding factor acute myeloid leukemia (CBF-AML). However, evidence for the efficacy of GO in this particular subgroup is primarily based on meta-analytic data from different trials conducted more than a decade ago. In this registry-based study, we evaluated the impact of adding GO to IC in 265 CBF-AML patients from the SAL, AMLCG, and CELL cooperative study groups. Patients receiving GO had a 2-year overall survival of 90% compared with 80% in those without GO (hazard ratio [HR] 0.45, 95% confidence interval [CI] 0.21-0.95, P = 0.036) and a 2-year event-free survival of 51% versus 36% (HR 0.69, 95% CI 0.48-0.99, P = 0.046). While complete remission rates in GO vs. non-GO patients were comparable (89% vs. 90%, P = 0.81), more GO patients achieved measurable residual disease-negative remission (77% vs. 49%, P < 0.001), resulting in numerically reduced cumulative incidence of relapse (HR 0.67, 95% CI 0.43-1.02, P = 0.06). Despite delayed platelet recovery, high-grade toxicities were not increased in GO-treated patients. These findings support the integration of GO into treatment protocols for IC-eligible patients with CBF-AML.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: TS received honorarium, financial support, and congress participation from Pfizer and served as a consultant for Pfizer. FM received support for meeting attendance from Servier, AbbVie, Incyte, Gilead, Jazz Pharmaceuticals, Novartis, Teva, Pfizer, and Amgen; received support for medical writing from Servier, and Springer Verlag; received research grants from Apis Technologies, and Daiichi Sankyo; received speaker honoraria from Servier, Jazz Pharmaceuticals, Daiichi Sankyo, and AbbVie; participated in advisory boards for Otsuka Pharma, Servier, Pfizer, and Daiichi Sankyo. SK received honorarium as a speaker from Eickeler and travel support from AbbVie, Alexion, and Jazz. KS received speaker honoraria and/or travel grants from and/or has been a consultant to: AbbVie, Alexa, Bristol Myers Squibb, Jazz Pharmaceuticals, Leo, Pfizer, Sanofi, Servier, Sobi, Stemline, Takeda, MSD, Gilead. GL received research grants not related to this manuscript from AGIOS, AQUINOX, AstraZeneca, Bayer, Celgene, Gilead, Janssen, MorphoSys, Novartis, F. Hoffmann-La Roche Ltd, and Verastem. GL received honoraria not related to this study from ADC Therapeutics, AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, BMS, Celgene, Constellation, Genase, Genmab, Gilead, Hexal/Sandoz, Immagene, Incyte, Janssen, Karyopharm, Lilly, Miltenyi, MorphoSys, MSD, NanoString, Novartis, PentixaPharm, Pierre Fabre, F. Hoffmann-La Roche Ltd, and Sobi. HCR received consulting and lecture fees from AbbVie, Roche, KinSea, Vitis, Cerus, Lilly, Novartis, Takeda, AstraZeneca, Vertex, and Merck. HCR received research funding from AstraZeneca and Gilead Pharmaceuticals. HCR is a co-founder of CDL Therapeutics GmbH. MBo has no conflict of interest regarding this work. Outside this study, he has received lecture fees and served on advisory boards for Jazz, MSD, and ActiTrexx. CR has received honoraria from AbbVie, Astellas, Bristol-Meyer-Squibb, Daiichi Sankyo, Jazz, Janssen, Novartis, Otsuka, Pfizer, Servier, and institutional research funding from AbbVie, Astellas, Novartis, Pfizer. CS received honoraria/has served on Advisory Boards for AbbVie, Astellas, AstraZeneca, BMS, Daiichi Sankyo, Laboratories Delbert, Jazz Pharmaceuticals, Novartis, Otsuka, Pfizer, Roche. CS received institutional research support from Jazz Pharmaceuticals and congress/travel grants from AbbVie, BMS, Jazz Pharmaceuticals, and Pfizer. Ethics approval and consent to participate: All procedures were performed in accordance with the relevant guidelines and regulations. The bio-registry of the SAL (NCT03188874), which encompasses 59 centers specialized in the treatment of hematologic neoplasms across Germany and the Czech Republic, is approved by the ethics committee of the Technical University Dresden (EK 98032010). The registry at the University Hospital Hamburg-Eppendorf is approved by the ethics committee of the Medical Association Hamburg (PV3469). Written informed consent was obtained from all participants.
Figures

References
-
- Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol J Am Soc Clin Oncol. 2011;29:369–77. - PubMed
-
- Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15:986–96. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

