Therapeutic impact of human trophoblast stem cells in peritoneal and pneumonia-induced sepsis in mice
- PMID: 40691621
- PMCID: PMC12282005
- DOI: 10.1186/s13287-025-04479-z
Therapeutic impact of human trophoblast stem cells in peritoneal and pneumonia-induced sepsis in mice
Abstract
Background: Sepsis is a complex and life-threatening disease process related to a systemic response to severe infection. Due to the challenges of treating patients with sepsis, new therapies are being investigated, including cell-based approaches. Trophoblast stem cells (TSCs) are immune privileged cells with immunomodulatory properties. Thus, we proposed that TSCs may be beneficial in experimental models of sepsis to regulate the immune response and curtail organ injury.
Methods: Sepsis was induced by experimental models in mice; cecal ligation and puncture (CLP) and lung infection with Streptococcus (S.) pneumoniae. TSCs were isolated from the chorionic villi of human (h) term placentas, and from mouse (m) placentas using anti-CD117 MicroBeads, and were administered intravenously 6 h after CLP or S. pneumoniae infection. We assessed mortality, bacterial clearance, organ injury, inflammatory response, and production of cytokines and chemokines.
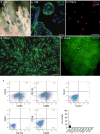
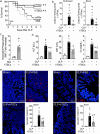
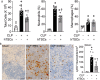
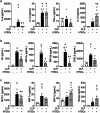
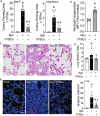
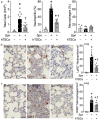
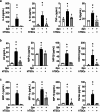
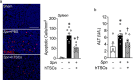
Results: CD117+ hTSCs did not express human leukocyte antigen (HLA) I or II, and were clonogenic and self-renewing. CLP led to severe mortality by 7 days, and administration of either hTSCs or mTSCs resulted in markedly improved survival compared with control cells or vehicle. hTSCs promoted bacterial clearance and decreased organ injury in the liver, kidney, spleen, and bowel. The elevated innate immune response in the peritoneum, predominantly neutrophils, was attenuated by hTSCs. In addition, neutrophil infiltration into the spleen was less in mice receiving hTSCs, which corresponded with reduced plasma pro-inflammatory cytokines and chemokines. When assessing the lung response to S. pneumoniae infection, administration of hTSCs resulted in fewer bacteria in bronchoalveolar lavage fluid (BALF) and lung tissue, and less lung edema and injury. Neutrophils, which were markedly increased in BALF, were diminished and infiltration of neutrophils and macrophages into the lungs was decreased by hTSCs. BALF pro-inflammatory cytokines and chemokines were mitigated by hTSCs to levels of Sham mice, and systemic injury to the liver and spleen was attenuated.
Conclusions: CD117+ hTSCs are immune privileged cells that when given after the onset of experimental models of infection/sepsis resulted in improved outcomes due to enhanced bacterial clearance, resolving inflammation, and less organ injury. These data support hTSCs as a potential cell-based therapy for sepsis.
Keywords: Acute organ injury; Bacterial clearance; Inflammation; Sepsis; Trophoblast stem cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Human Cells: The trophoblast stem cells were harvested from human term placental tissue. All of the tissue was from discarded placental specimens, de-identified prior to collection by the research team. Thus, the study was considered to be Not Human Subjects Research by the Brigham and Women’s Hospital Institutional Review Board (Record ID 1830). Animal Ethics declaration: Studies using mice were carried out in accordance with the Public Health Service policy on the humane care and use of laboratory animals, and approved by the Institutional Animal Care and Use Committee (IACUC) of Brigham and Women’s Hospital (Protocol 2016N000308). As per our IACUC protocol, with major surgical procedures, analgesics were used. Moreover, the use of humane endpoints was applied during the survival experiments in the CLP model of sepsis. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the united states: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. - PubMed
-
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. - PubMed
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–51. - PubMed
-
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of Hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–72. - PubMed
-
- Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the united States 2003 to 2007. Crit Care Med. 2012;40(3):754–61. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

